Abstract
Objectives
To compare block sequential regularized expectation maximization (BSREM) and ordered subset expectation maximization (OSEM) for the detection of in-transit metastasis (ITM) of malignant melanoma in digital [18F]FDG PET/CT.
Methods
We retrospectively analyzed a cohort of 100 [18F]FDG PET/CT scans of melanoma patients with ITM, performed between May 2017 and January 2020. PET images were reconstructed with both OSEM and BSREM algorithms. SUVmax, target-to-background ratio (TBR), and metabolic tumor volume (MTV) were recorded for each ITM. Differences in PET parameters were analyzed with the Wilcoxon signed-rank test. Differences in image quality for different reconstructions were tested using the Man-Whitney U test.
Results
BSREM reconstruction led to the detection of 287 ITM (39% more than OSEM). PET parameters of ITM were significantly different between BSREM and OSEM reconstructions (p < 0.001). SUVmax and TBR were higher (76.5% and 77.7%, respectively) and MTV lower (49.5%) on BSREM. ITM missed with OSEM had significantly lower SUVmax (mean 2.03 vs. 3.84) and TBR (mean 1.18 vs. 2.22) and higher MTV (mean 2.92 vs. 1.01) on OSEM compared to BSREM (all p < 0.001).
Conclusions
BSREM detects significantly more ITM than OSEM, owing to higher SUVmax, higher TBR, and less blurring. BSREM is particularly helpful in small and less avid lesions, which are more often missed with OSEM.
Key Points
• In melanoma patients, [ 18 F]FDG PET/CT helps to detect in-transit metastases (ITM), and their detection is improved by using BSREM instead of OSEM reconstruction.
• BSREM is particularly useful in small lesions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cutaneous malignant melanoma (CMM) is the 5th most common cancer in men and the 6th most common cancer in women worldwide [1, 2]. The incidence of CMM increased in the last 40 years, partly attributable to improved screening programs, with approximately 287,700 new annual cases globally [3].
Cutaneous and subcutaneous melanoma metastases are very frequent and include microsatellite, satellite, and in-transit metastases (ITMs). With the 8th edition of the American Joint Committee on Cancer (AJCC), these three different entities were merged into the single subcategory “c” of the N classification (N1c, N2c, and N3c) [4]. The association of such metastases with poor prognosis was demonstrated by several studies [5,6,7,8,9]. In particular, ITMs occur in 2–10% of melanoma patients and are frequently associated with the development of nodal and/or systemic metastases [10], even in sentinel node-negative patients [11]. In 2015, Beasley et al [12] have reported a 5-year survival rate of 59% in patients without regional nodal disease compared to 19% for those with nodal disease (including ITM).
To reduce melanoma-related mortality and distant metastasis development, an earlier detection of ITM could be helpful, although no such data exists currently. Moreover, ITM can be treated both with novel systemic agents (as immune checkpoint inhibitors and mitogen-activated protein kinase pathway inhibitors) and with locoregional interventions (as surgery, electrochemotherapy, isolated limb infusion or perfusion, and oncolytic viral therapy) [13,14,15,16,17,18].
Typically, ITMs are detected during clinical examination of patients or by ultrasound (US) using high-frequency (HF) probes, which is time-consuming, operator-dependent, and limited in terms of tissue depth and small-sized lesions. 2-Deoxy-2-[18F]fluoro-D-glucose positron emission tomography/computed tomography ([18F]FDG PET/CT) is typically used for the staging and restaging of high-risk melanoma patients, mainly for the detection of lymph node metastases and distant metastases. With the advent of digital PET and novel iterative reconstruction techniques, the detectability of small-sized [19] and faintly [18F]FDG-avid lesions has improved considerably [20, 21]. Hence, digital PET/CT may play a relevant role in the detection of the exact number, size, and location of ITM and may subsequently impact patient management and therapy-related decisions [18, 22,23,24]. The aim of our study was to assess the value of [18F]FDG PET images reconstructed with block sequential regularized expectation maximization (BSREM) compared to the clinical standard ordered subset expectation maximization (OSEM) for ITM detection.
Material and methods
Patient selection
We retrospectively analyzed a cohort of 1575 consecutive examinations of patients, who underwent a clinically indicated [18F]FDG PET/CT scan on a digital scanner for the staging/restaging of malignant melanoma at the University Hospital of Zurich between May 2017 and January 2020. Only patients with documented willingness to the use of their medical data for research were included (423 examinations excluded) in this retrospective, observational study. Our study was approved by the local ethics committee and was conducted in compliance with ICH-GCP rules and the Declaration of Helsinki. All reports of the remaining 1152 PET/CT scans were reviewed for reported ITM presence. In each reported case, the ITM presence on imaging was verified by one doubly board-certified radiologist/nuclear medicine physician with 12 years of experience in oncological hybrid imaging (M.H.).
Hence, eligible patients matched all the following inclusion criteria: (a) histologically proven melanoma; (b) presence of at least one in-transit metastasis described in report (based on BSREM algorithm) and verified by the above-mentioned reader; (c) PET/CT scan acquired on a digital scanner with silicon photomultiplier (SiPM) technology; (f) availability of both OSEM and BSREM reconstructions. The final study cohort consisted of a total of 100 examinations.
At our institution, BSREM serves as clinical standard for all oncological [18F]FDG PET exams carried out on digital scanners, and OSEM is reconstructed by default in order to ensure comparability with analog scanners without BSREM technology. Pathological confirmation, clinical examination including ultrasound, and outcome and/or imaging after 3–6 months served as the standard of reference for proving ITM. In 65 of the 100 PET/CT scans, at least one ITM was histologically proven. In the remaining 35 cases, the location of the lesion (between the primary site and regional nodal basin, along the lymphatic stream), clinical examination including ultrasound, and outcome and/or imaging after 3–6 months served as the standard of reference for the designation of a lesion as ITM.
PET/CT acquisition
All included patients underwent a PET/CT scan on a digital scanner with SiPM technology (GE Discovery Molecular Insights - DMI PET/CT, GE Healthcare). The injected tracer activity was 221.53 ± 6.67 MBq of [18F]FDG. After an uptake time of 60 min and following CT acquisition both for attenuation correction and anatomical correlation, PET data were acquired in 3-dimensional time-of-flight (TOF) mode, covering the identical anatomical region of the CT.
PET image datasets were reconstructed with different standardized settings (all with a 256 × 256 pixel matrix):
-
1-
OSEM: 3 iterations, 16 subsets, FWHMI of 6.3 mm, 1:4 Z-axis filter, and 6.4-mm Gaussian filter with both time-of-flight (TOF) and point spread function (PSF) modeling (OSEMPSF; VUE Point FX with SharpIR, GE Healthcare).
-
2-
BSREM (Q.Clear, GE Healthcare) with both TOF and PSF and a β-value of 450 (BSREM450) which represents the institutional standard [25,26,27,28].
Quantitative imaging analysis
Quantitative analysis was performed by two readers, blinded to clinical data. Readers were provided with de-identified images reconstructed with OSEM and BSREM, in random patient and reconstruction order. The task of in-transit metastasis detection was assigned to the readers, and readers recorded the slice position and SUVmax of all lesions detected. PET images were segmented using a dedicated workstation (GE Healthcare). The following indices were recorded for each lesion: location, metabolic tumor volume (MTV), maximum standardized uptake value (SUVmax), and mean standardized uptake value (SUVmean).
PET parameters were measured on PET images using a volume of interest (VOI) including the whole lesion volume, outlined with a 3D semi-automatic contouring tool, and applying a threshold set at 42% of the SUVmax. Target-to-background ratios (TBR), defined as the ITM SUVmax corrected for physiological blood pool SUVmean, were also calculated [29]. All quantitative image analyses were performed on both OSEM and BSREM reconstructions, using cloned VOIs for both reconstructions.
Statistical analysis
Categorical variables are expressed as proportions, and continuous variables are presented as mean ± standard deviation (SD) or median (range), depending on the distribution of values. We assessed the number of ITM detected with either reconstruction algorithm. Moreover, we assessed the frequency of PET parameter changes (SUVmax, TBR, and MTV) comparing BSREM with OSEM. Based on PET Response Criteria in Solid Tumors (PERCIST), we considered a change in PET parameters of ± 30% (BSREM vs. OSEM) as clinically relevant [30]. The Wilcoxon signed-rank test was used to test for differences in lesional PET parameters among both reconstructions. Differences in image quality among reconstructions were tested using the Mann-Whitney U test and the Pearson test, with linear regression used to calculate the correlation coefficients. Statistical significance was considered for p < 0.05. Statistical analyses were performed using SPSS version 26.0 (IBM) [31].
Results
Patient and tumor characteristics
Patient and tumor characteristics are listed in Table 1 and supplemental table S1. Eighty-eight percent of the scans were performed for restaging, and 12% for initial staging purposes. Before PET/CT, all patients had already undergone surgery (100%; primary tumor and/or lymph node surgery) and several other treatments, such as chemotherapy (4%), small molecule targeted therapy (15%), immunotherapy (47%), radiotherapy (21%), TVEC (10, and other therapies (19%).
In our study, the mean follow-up time after the analyzed PET/CT scan was 18.2 ± 1.5 (0.0–131.0) months. Fifty-four PET/CT scans detected only ITM but no nodal metastases, 14 PET/CT scans detected ITM and lymph node metastases, and 32 PET/CT scans detected ITM and distant metastasis. According to RECIST 1.1 criteria, favorable outcome (complete response + partial response + stable disease) was higher in patients with only ITM (34/54; 34% of the entire cohort) compared to patients with ITM and lymph node metastases (10/14; 10%) and patients with ITM and distant metastases (15/32; 15%).
Differences in PET parameters between OSEM and BSREM algorithms
Lesions suspected to represent ITM were detected by the readers in all 100 PET/CT scans included in this study. The majority of ITM (69.0%) were located in the legs, 16.4% in the torso, 8.0% in the arms, and 6.6% in the head and neck.
Readers detected a total of 295 lesions with BSREM, 214 with OSEM. Overall, 8 of the detected lesions turned out false positive (6 granulomatous inflammations, 2 lymph node metastases, all confirmed by histopathology) in 8 subjects, who underwent a restaging PET/CT scan. All of these lesions were recorded by the readers with both BSREM and OSEM. All 8 false-positive lesions were present in subjects who had also true positive lesions, i.e., ITM. Interestingly, all these false-positive lesions were also initially considered clinically to represent ITM.
Using BSREM, readers correctly detected a total of 287 ITM, of which 206 were detected also with OSEM (difference of 39%), equaling a mean per patient ITM number of 2.06 for OSEM and 2.87 for BSREM (p < 0.001). In 20 PET/CT scans (20% of cohort), ITM presence was detected only by BSREM, but not by OSEM. With BSREM/OSEM, the number of in-transit metastases detected per patient was 1 lesion (42% / 25% of cases, respectively), 2–5 lesions (44%/41%), and > 5 lesions (14%/14%).
All PET parameters (SUVmax, TBR, MTV) were significantly different between BSREM and OSEM reconstructions (p < 0.001), both for the entire cohort and for several sub-groups, such as ITM detected only with BSREM (retrospectively analyzed also with OSEM) and ITM in different anatomical locations (head and neck, torso, arms, and legs respectively). In the entire cohort, there was a difference (BSREM vs. OSEM; all p < 0.001) in ITM SUVmax of +76.5% (mean 8.42 vs. 4.77), TBR of +77.7% (mean 4.78 vs. 2.69), and MTV of - 49.5% (mean 1.01 vs. 2.00 cm3). Figure 1 shows the box plots of ITM PET parameters in OSEM and BSREM, respectively.
The latter result is consistent with the fact that BSREM detects smaller lesions. As shown in Table 2, all these differences were more pronounced for ITM that were missed with OSEM reconstruction and retrospectively analyzed. Here, we observed an increase in ITM SUVmax by +89.2% from OSEM to BSREM (mean 2.03 vs. 3.84, respectively, p < 0.001), in ITM TBR by +88.1% (mean 1.18 vs. 2.22, respectively, p < 0.001), and a decrease in ITM MTV by -65.4% (mean 2.92 vs. 1.01 cm3, respectively, p < 0.001).
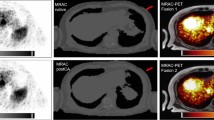
SUVmax of ITM in BSREM and OSEM according to the anatomical location are given in Fig. 2. The highest differences in SUVmax between BSREM and OSEM were found in the head/neck and in the legs, as shown in Table 3. One representative example is given in Fig. 3.
[18F]FDG PET/CT of a malignant melanoma patient with three right-sided lower leg in-transit metastases, visible on maximum intensity projection (MIP) images (a, arrow) as well as on axial CT and PET images (b–d, white arrows), better defined by BSREM reconstruction compared to OSEM reconstruction. BSREM reconstruction also yielded higher SUVmax, lower MTV, and better noise characteristics compared to OSEM reconstruction, as indicated by axial PET images (b–d)
We found significant differences in PET parameters between subjects with low and high BMI (cut-off 25), both for OSEM and BSREM (p < 0.001), as shown in supplemental table S2. Moreover, we found a negative correlation between BMI and ITM SUVmax and TBR, and a positive correlation between BMI and blood pool SUVmean and ITM MTV, more pronounced on BSREM than on OSEM (supplemental table S3).
Discussion
Our study is the first one (to the best of our knowledge) reporting improved detection of in-transit metastases with BSREM compared to the clinical gold standard OSEM. The major findings of our study are as follows: (1) BSREM leads to the detection of more ITM than OSEM (+39% more); (2) all ITM PET parameters (SUVmax, TBR, MTV) are significantly different between BSREM and OSEM (p < 0.001), with an SUVmax increase by 76.5%, a TBR increase by 77.7%, and a MTV decrease by 49.5% from OSEM to BSREM.
Several studies have highlighted the role of [18F]FDG PET/CT in patients with advanced-stage melanoma, especially for the detection of distant metastases during follow-up (sensitivity 82–100% and specificity 45–100%), leading to treatment change in 13–74% of stage III/IV patients [32]. As recently highlighted by Laudicella et al [33], digital PET systems and new reconstruction algorithms lead to a more accurate diagnosis, staging, and therapeutic evaluation of melanoma patients through better image quality, higher spatial resolution, and more accurate image reconstruction. Aljared et al [34] reported added value of BSREM reconstruction in a melanoma patient, where four [18F]FDG-avid ITMs were detected only on BSREM reconstruction. It was assumed that BSREM may have implications for the detection of small lesions [35], such as ITM, and might influence therapeutic decisions.
Our study’s results validate this hypothesis: BSREM identified 81 ITM more than OSEM (287 versus 206; +39%). In 20 patients, the presence of ITM was detected only with BSREM, but missed with OSEM. This can be attributed to the fact that focal uptake with higher activity concentration tends to converge faster compared to the uptake with lower activity concentration, so the convergence benefit of BSREM reconstruction in providing more convergent foci compared to OSEM [20, 23].
Thereof, a single lesion was detected in 17 cases and 2 to 5 lesions were detected in 3 cases. BSREM lead to the identification of 1 lesion with an uptake below blood pool background (SUVmax 0.84 and TBR 0.48), 18 lesions with MTV < 0.1 cm3, and 2 lesions with MTV = 0.02 cm3, corresponding to a diameter of approximately 3 mm, which is in line with Baratto et al [36], who detected lesions < 0.7 cm with digital PET systems (Fig. 4). Moreover, MTV was increasing particularly with OSEM in lesions with low SUVmax, translating into the observed blurring effect with OSEM, and such may serve as a measure of poor detectability. This also implies that MTV is overestimated using OSEM, particularly in small lesions.
[18F]FDG PET/CT of a malignant melanoma patient with several left-sided leg in-transit metastases, visible on MIP images (a). Of these, the two proximal ones (black arrows) were detected only at BSREM reconstruction. As shown in the axial images (b and c), BSREM yielded higher SUVmax, lower MTV, and better noise characteristics compared to OSEM. Despite their high uptake at BSREM and the location highly suspicious for ITM, these two lesions had no anatomical correlate on CT (white arrows), except for a slight skin thickening at one site. Both lesions were subsequently confirmed by histopathology
Patients presenting with ITM on clinical examination should undergo restaging including physical examination and whole-body imaging in order to guide therapeutic options [12]. [18F]FDG PET/CT allows both ITM detection and whole-body restaging at the same time. Moreover, the identification of the exact number and site of in-transit metastases is fundamental for the choice of the optimal therapy: ITMs are typically resected if less than 3–4 lesions and none larger than 5 cm; otherwise, locoregional treatment should be evaluated, with a preference for TVEC in the torso or head/neck ITM. Systemic therapy should be chosen with a concurrent clinically evident metastatic or nodal disease with or without the aforementioned simultaneous specific ITM treatment [16, 35]. In these terms, the positive impact that BSREM, comparing to OSEM reconstruction, could have in the evaluation of [18F]FDG PET/CT images is evident.
Obviously, future prospective and comparative studies with other reference methods for ITM detention are needed to determine the added value of [18F]FDG PET/CT in evaluating ITMs in a clinical setting and to analyze possible associations between ITM number and site with recurrence-free survival (RFS), distant metastasis-free survival (DMFS), and melanoma-specific survival. In 2014, Solivetti et al [37] studied whether US could be replaced or integrated with other techniques, such as [18F]FDG PET/CT and telethermography (TT). All 52 ITMs in 15 patients in their study were detected by HF-US (100%), 24/52 were detected by PET/CT (42.6%), and 15/52 were detected by TT (27.7%). PET/CT reported 3.7% false positives, while no false positives were reported by TT. Our study did not aim to compare different examination techniques; however, we hypothesize that these results may be different in a larger cohort of patients and with the use of new digital PET/CT systems. In our cohort, only 8 out of 287 lesions finally resulted to be false positive (0.96% of cases).
Finally, the impact of [18F]FDG PET/CT should be evaluated considering also the contextual whole-body (re)staging, which is compulsory after ITM detection. Two different retrospective studies on two large cohorts of melanoma patients with ITM (380 German and 11614 Australian patients, respectively) found that lymph node involvement is an important prognostic factor in this cohort [38, 39]. Even if it was not the aim of our study, we reported similar results with a higher favorable outcome in patients with only ITM (34/54) compared to patients with ITM and lymph node metastases (10/14) and patients with ITM and distant metastases (15/32). Also in this context, we expect that further studies will assess the benefit of BSREM reconstruction in the evaluation of the global tumor burden in malignant melanoma. Such would allow for a more accurate assessment of the state of the disease, taking into account the whole-body tumor burden, and its impact on staging and follow-up.
Our study is not exempt from limitations. First, although readers were blinded to the type of reconstruction used, an experienced reader may recognize the actual algorithm used based on the reconstructed images. Second, not all ITMs were proven by histology. However, all lesions were located in the subcutaneous adipose tissue between the primary tumor site and the regional nodal basin, which is suggestive for ITM, and lesions were also suspected to represent ITM on ultrasound. It is known that rarely also ectopic lymph nodes may exist in the subcutaneous adipose tissue. Hence, some of the “ITMs” could actually have represented lymph node metastases, which in turn even would have led to an upstaging of patients. Notably, lesions suspicious for ITM were not detected in anatomical regions other than the one that harbored the primary tumor in our cohort. Since PET/CT intrinsically represents a standard of reference for ITM detection, we cannot comment on false-negative lesions. However, reporting diagnostic accuracy of PET was also not the thrust of our study, and such would require a comparison with ultrasound in order to make sense. Of note, in our cohort, no additional ITMs were detected with ultrasound besides the ones detected with PET. Third, the exclusion of melanoma patients scanned with analog PET/CT may have reduced the possibility to give epidemiology information about ITM frequency related to the entire patient population with malignant melanoma.
Conclusion
The detection of in-transit metastases in [18F]FDG PET/CT has significantly impacted by the use of BSREM reconstruction. BSREM detects significantly more (+39%) in-transit metastases than OSEM, with a significant difference (all p < 0.001) in ITM SUVmax (+76.5%), TBR (+77.7%), and MTV (- 49.5%) compared to OSEM.
Abbreviations
- [18F]FDG:
-
2-Deoxy-2-[18F]fluoro-D-glucose
- AJCC:
-
American Joint Committee on Cancer
- BMI:
-
Body mass index
- BPL:
-
Bayesian penalized likelihood
- BRAF:
-
v-Raf murine sarcoma viral oncogene homolog B
- BSREM:
-
Block sequential regularized expectation maximization
- CECT:
-
Contrast-enhanced computed tomography
- CMM:
-
Cutaneous malignant melanoma
- CT:
-
Computed tomography
- ITM:
-
In-transit metastases
- MEK:
-
Mitogen-activated protein kinase
- MIP:
-
Maximum intensity projection
- MR:
-
Magnetic resonance
- OSEM:
-
Ordered subset expectation maximization
- PET:
-
Positron emission tomography
- PSF:
-
Point spread function
- SiPM:
-
Silicon photomultiplier
- SUVmax:
-
Maximum standardized uptake value
- TOF:
-
Time of flight
- TVEC:
-
Talimogene laherparepvec
- VOI:
-
Volume of interest
References
Tripp MK, Watson M, Balk SJ et al (2016) State of the science on prevention and screening to reduce melanoma incidence and mortality: the time is now. CA Cancer J Clin 66:460–480. https://doi.org/10.3322/caac.21352
Schadendorf D, Fisher DE, Garbe C et al (2015) Melanoma. Nat Rev Dis Primers 1. https://doi.org/10.1038/nrdp.2015.3
Ferlay J, Colombet M, Soerjomataram I et al (2019) Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 144:1941–1953
Gershenwald JE, Scolyer RA (2018) Melanoma Staging: American Joint Committee on Cancer (AJCC) 8th Edition and Beyond. Ann Surg Oncol 25:2105–2110
Pararajasingam A, Goodwin R (2020) In-transit metastases: the migration of melanoma. Br J Hosp Med 81:1. https://doi.org/10.12968/hmed.2020.0202
Clemente-Ruiz de Almiron A, Serrano-Ortega S (2012) Factores de riesgo de metástasis en tránsito en pacientes con melanoma cutáneo. Actas Dermosifiliogr 103:207–213. https://doi.org/10.1016/j.ad.2011.06.002
Marcoval J, Ferreres JR, Penín RM et al (2011) Análisis descriptivo de los patrones de recidiva cutánea en los pacientes con melanoma. Actas Dermosifiliogr 102:791–796. https://doi.org/10.1016/j.ad.2011.04.006
Rao UNM, Ibrahim J, Flaherty LE et al (2002) Implications of microscopic satellites of the primary and extracapsular lymph node spread in patients with high-risk melanoma: Pathologic corollary of eastern cooperative oncology group trial E1690. J Clin Oncol 20:2053–2057. https://doi.org/10.1200/JCO.2002.08.024
León P, Daly JM, Synnestvedt M et al (1991) The prognostic implications of microscopic satellites in patients with clinical stage I melanoma. Arch Surg 126:1461–1468. https://doi.org/10.1001/archsurg.1991.01410360031006
Gershenwald JE, Scolyer RA, Hess KR et al (2017) Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 67:472–492. https://doi.org/10.3322/caac.21409
Ito K, Teng R, Schöder H et al (2019) 18 F-FDG PET/CT for monitoring of ipilimumab therapy in patients with metastatic melanoma. J Nucl Med 60:335–341. https://doi.org/10.2967/jnumed.118.213652
Beasley G, Tyler D (2014) In-transit Melanoma Metastases: Incidence, Prognosis, and the Role of Lymphadenectomy. Ann Surg Oncol 22:358–360
Testori A, Ribero S, Bataille V (2017) Diagnosis and treatment of in-transit melanoma metastases. Eur J Surg Oncol 43:544–560
Nan Tie E, Lai-Kwon J, Rtshiladze MA et al (2020) Efficacy of immune checkpoint inhibitors for in-transit melanoma. J Immunother Cancer 8. https://doi.org/10.1136/jitc-2019-000440
Nadler A, Look Hong NJ, Alavi N et al (2020) Lesional therapies for in-transit melanoma. J Surg Oncol. https://doi.org/10.1002/jso.26121
Wright FC, Kellett S, Look Hong NJ et al (2020) Locoregional management of in-transit metastasis in melanoma: an Ontario Health (Cancer Care Ontario) Clinical Practice Guideline. Curr Oncol 27:e318–e325. https://doi.org/10.3747/co.27.6523
Bomar L, Senithilnathan A, Ahn C (2019) Systemic therapies for advanced melanoma. Dermatol Clin 37:409–423
Burns D, George J, Aucoin D et al (2019) The pathogenesis and clinical management of cutaneous melanoma: an evidence-based review. J Med Imaging Radiat Sci 50:460–469.e1
Liberini V, Kotasidis F, Treyer V et al (2021) Impact of PET data driven respiratory motion correction and BSREM reconstruction of 68Ga-DOTATATE PET/CT for differentiating neuroendocrine tumors (NET) and intrapancreatic accessory spleens (IPAS). Sci Rep 11:2273. https://doi.org/10.1038/s41598-020-80855-4
Messerli M, Kotasidis F, Burger IA et al (2019) Impact of different image reconstructions on PET quantification in non-small cell lung cancer: a comparison of adenocarcinoma and squamous cell carcinoma. Br J Radiol 92. https://doi.org/10.1259/bjr.20180792
Messerli M, Stolzmann P, Egger-Sigg M et al (2018) Impact of a Bayesian penalized likelihood reconstruction algorithm on image quality in novel digital PET/CT: clinical implications for the assessment of lung tumors. EJNMMI Phys 5:27. https://doi.org/10.1186/s40658-018-0223-x
Groen LC, Lazarenko SV, Schreurs HW, Richir MC (2019) Evaluation of PET/CT in patients with stage III malignant cutaneous melanoma. Am J Nucl Med Mol Imaging 9:168–175
Lantos J, Mittra ES, Levin CS, Iagaru A (2018) Standard OSEM vs. regularized PET image reconstruction: qualitative and quantitative comparison using phantom data and various clinical radiopharmaceuticals. Am J Nucl Med Mol Imaging 8:110–118
Hatami S, Frye SA, McMunn A, et al (2020) Added Value of Digital Over Analog PET/CT: More Significant as Image Field of View (FOV) and Body Mass Index (BMI) Increases. J Nucl Med Technol jnmt.120.244160. https://doi.org/10.2967/jnmt.120.244160
Trägårdh E, Minarik D, Almquist H et al (2019) Impact of acquisition time and penalizing factor in a block-sequential regularized expectation maximization reconstruction algorithm on a Si-photomultiplier-based PET-CT system for 18F-FDG. EJNMMI Res 9:64. https://doi.org/10.1186/s13550-019-0535-4
Lindström E, Sundin A, Trampal C et al (2018) Evaluation of penalized-likelihood estimation reconstruction on a digital time-of-flight PET/CT scanner for 18 F-FDG whole-body examinations. J Nucl Med 59:1152–1158. https://doi.org/10.2967/jnumed.117.200790
Shkumat NA, Vali R, Shammas A (2020) Clinical evaluation of reconstruction and acquisition time for pediatric 18F-FDG brain PET using digital PET/CT. Pediatr Radiol 50:966–972. https://doi.org/10.1007/s00247-020-04640-1
Sah BR, Stolzmann P, Delso G et al (2017) Clinical evaluation of a block sequential regularized expectation maximization reconstruction algorithm in 18F-FDG PET/CT studies. Nucl Med Commun 38:57–66. https://doi.org/10.1097/MNM.0000000000000604
Boellaard R, Delgado-Bolton R, Oyen WJG et al (2015) FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 42:328–354
Wahl RL, Jacene H, Kasamon Y, Lodge MA (2009) From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J Nucl Med 50:122S
IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.
Bisschop C, de Heer EC, Brouwers AH et al (2020) Rational use of 18F-FDG PET/CT in patients with advanced cutaneous melanoma: a systematic review. Crit Rev Oncol Hematol 153
Laudicella R, Baratto L, Minutoli F et al (2019) Malignant cutaneous melanoma: updates in PET imaging. Curr Radiopharm 13:14–23. https://doi.org/10.2174/1874471012666191015095550
Aljared A, Alharbi AA, Huellner MW (2018) BSREM reconstruction for improved detection of in-transit metastases with digital FDG-PET/CT in patients with malignant melanoma. Clin Nucl Med 43:370–371. https://doi.org/10.1097/RLU.0000000000002024
Perone JA, Farrow N, Tyler DS, Beasley GM (2018) Contemporary approaches to in-transit melanoma. J Oncol Pract 14:292–300
Baratto L, Park SY, Hatami N et al (2017) 18F-FDG silicon photomultiplier PET/CT: A pilot study comparing semi-quantitative measurements with standard PET/CT. PLoS One 12. https://doi.org/10.1371/journal.pone.0178936
Solivetti FM, Desiderio F, Guerrisi A et al (2014) HF ultrasound vs PET-CT and telethermography in the diagnosis of in-transit metastases from melanoma: a prospective study and review of the literature. J Exp Clin Cancer Res 33
Read RL, Haydu L, Saw RPM et al (2015) In-transit melanoma metastases: incidence, prognosis, and the role of lymphadenectomy. Ann Surg Oncol 22:475–481. https://doi.org/10.1245/s10434-014-4100-0
Weide B, Faller C, Büttner P et al (2013) Prognostic factors of melanoma patients with satellite or in-transit metastasis at the time of stage III diagnosis. PLoS One 8. https://doi.org/10.1371/journal.pone.0063137
Acknowledgements
We would like to express our gratitude to the anonymous patients on whom this work is based and the staff of the Department of Nuclear Medicine for their excellent technical support.
Funding
Open Access funding provided by Universität Zürich. This study has received funding by a specific IIS grant from GE Healthcare. The University Hospital Zurich holds a research agreement with GE Healthcare (unrelated to the current study).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Martin Huellner, PD, MD.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: MH is a recipient of grants and speaker’s fees from GE Healthcare, grants for translational and clinical cardiac and oncological research from the Alfred and Annemarie von Sick Grant legacy, and grants from the Artificial Intelligence in oncological Imaging Network by the University of Zurich.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective
• Diagnostic or prognostic study
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 1424 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liberini, V., Messerli, M., Husmann, L. et al. Improved detection of in-transit metastases of malignant melanoma with BSREM reconstruction in digital [18F]FDG PET/CT. Eur Radiol 31, 8011–8020 (2021). https://doi.org/10.1007/s00330-021-07852-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-07852-7