Abstract
Objectives
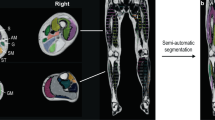
Magnetic resonance imaging (MRI) constitutes a powerful outcome measure in neuromuscular disorders, yet there is a broad diversity of approaches in data acquisition and analysis. Since each neuromuscular disease presents a specific pattern of muscle involvement, the recommended analysis is assumed to be the muscle-by-muscle approach. We, therefore, performed a comparative analysis of different segmentation approaches, including global muscle segmentation, to determine the best strategy for evaluating disease progression.
Methods
In 102 patients (21 immune-mediated necrotizing myopathy/IMNM, 21 inclusion body myositis/IBM, 10 GNE myopathy/GNEM, 19 Duchenne muscular dystrophy/DMD, 12 dysferlinopathy/DYSF, 7 limb-girdle muscular dystrophy/LGMD2I, 7 Pompe disease, 5 spinal muscular atrophy/SMA), two MRI scans were obtained at a 1-year interval in thighs and lower legs. Regions of interest (ROIs) were drawn in individual muscles, muscle groups, and the global muscle segment. Standardized response means (SRMs) were determined to assess sensitivity to change in fat fraction (ΔFat%) in individual muscles, muscle groups, weighted combinations of muscles and muscle groups, and in the global muscle segment.
Results
Global muscle segmentation gave high SRMs for ΔFat% in thigh and lower leg for IMNM, DYSF, LGMD2I, DMD, SMA, and Pompe disease, and only in lower leg for GNEM and thigh for IBM.
Conclusions
Global muscle segment Fat% showed to be sensitive to change in most investigated neuromuscular disorders. As compared to individual muscle drawing, it is a faster and an easier approach to assess disease progression. The use of individual muscle ROIs, however, is still of interest for exploring selective muscle involvement.
Key Points
• MRI-based evaluation of fatty replacement in muscles is used as an outcome measure in the assessment of 1-year disease progression in 8 different neuromuscular diseases.
• Different segmentation approaches, including global muscle segmentation, were evaluated for determining 1-year fat fraction changes in lower limb skeletal muscles.
• Global muscle segment fat fraction has shown to be sensitive to change in lower leg and thigh in most of the investigated neuromuscular diseases.




Similar content being viewed by others
Abbreviations
- AL:
-
Adductor longus
- AM:
-
Adductor magnus
- BF:
-
Biceps femoris
- DMD:
-
Duchenne muscle dystrophy
- DYSF:
-
Dysferlinopathy
- ED:
-
Extensor digitorum
- Fat%:
-
Fat fraction
- FIB:
-
Fibularis
- FOV:
-
Field of view
- GL:
-
Gastrocnemius lateralis
- GM:
-
Gastrocnemius medialis
- GNEM:
-
GNE myopathy
- GRA:
-
Gracilis
- HSTR:
-
Hamstring
- IBM:
-
Inclusion body myositis
- I-M FAT:
-
Intermuscular fat
- IMNM:
-
Immune-mediated necrotizing myopathy
- LEG_ANT:
-
Anterior part of leg
- LGMD2I:
-
Limb-girdle muscular dystrophy type 2I
- MSE:
-
Multi-spin-echo
- PER:
-
Peroneus
- QUAD:
-
Quadriceps
- RF:
-
Rectus femoris
- SAR:
-
Sartorius
- SM:
-
Semimembranosus
- SMA:
-
Spinal muscular atrophy
- SOL:
-
Soleus
- SRM:
-
Standardized response mean
- ST:
-
Semitendinosus
- TA:
-
Tibialis anterior
- TE:
-
Echo time
- TP:
-
Tibialis posterior
- TR:
-
Repetition time
- VI:
-
Vastus intermedius
- VL:
-
Vastus lateralis
- VM:
-
Vastus medialis
- ΔFat%:
-
Fat fraction change after one year
References
Carlier PG, Marty B, Scheidegger O et al (2016) Skeletal muscle quantitative nuclear magnetic resonance imaging and spectroscopy as an outcome measure for clinical trials. J Neuromuscul Dis 3:1–28
Triplett WT, Baligand C, Forbes SC et al (2014) Chemical shift-based MRI to measure fat fractions in dystrophic skeletal muscle. Magn Reson Med 72:8–19
Wokke BH, van den Bergen JC, Versluis MJ et al (2014) Quantitative MRI and strength measurements in the assessment of muscle quality in Duchenne muscular dystrophy. Neuromuscul Disord 24:409–416
Bonati U, Hafner P, Schädelin S et al (2015) Quantitative muscle MRI: a powerful surrogate outcome measure in Duchenne muscular dystrophy. Neuromuscul Disord 25:679–685
Wary C, Azzabou N, Giraudeau C et al (2015) Quantitative NMRI and NMRS identify augmented disease progression after loss of ambulation in forearms of boys with Duchenne muscular dystrophy. NMR Biomed 28:1150–1162
Gaeta M, Messina S, Mileto A et al (2012) Muscle fat-fraction and mapping in Duchenne muscular dystrophy: evaluation of disease distribution and correlation with clinical assessments preliminary experience. Skeletal Radiol 41:955–961
Willis TA, Hollingsworth KG, Coombs A et al (2013) Quantitative muscle MRI as an assessment tool for monitoring disease progression in LGMD2I: a multicentre longitudinal study. PLoS One 8:e70993
Murphy AP, Morrow J, Dahlqvist JR et al (2019) Natural history of limb girdle muscular dystrophy R9 over 6 years: searching for trial endpoints. Ann Clin Transl Neurol 6:1033–1045
Paradas C, Moore U, James M et al (2016) Clinical outcome study for dysferlinopathy: one-year follow-up. Neuromuscul Disord 26:92–93
Smith FE, Wilson I, Torron R et al (2017) Quantitative magnetic resonance imaging of the skeletal muscle in a multi-center dysferlinopathy study: two-year follow-up. Magn Reson Mater Phys Biol Med 30:63
Diaz-Manera J, Fernandez-Torron R, LLauger J et al (2018) Muscle MRI in patients with dysferlinopathy: pattern recognition and implications for clinical trials. J Neurol Neurosurg Psychiatry 89:1071–1081
Chabanon A, Seferian AM, Daron A et al (2018) Prospective and longitudinal natural history study of patients with type 2 and 3 spinal muscular atrophy: baseline data NatHis-SMA study. PLoS One 13:e0201004
Gidaro T, Reyngoudt H, Le Louër J et al (2020) Quantitative nuclear magnetic resonance imaging detects subclinical changes over 1 year in skeletal muscle of GNE myopathy. J Neurol 267:228–238
Carlier PG, Azzabou N, de Sousa PL et al (2015) Skeletal muscle quantitative nuclear magnetic resonance imaging follow-up of adult Pompe patients. J Inherit Metab Dis 38:565–572
Nunez-Peralta C, Alonso-Perez A, Llauger J et al (2020) Follow-up of late-onset Pompe disease patients with muscle magnetic resonance imaging reveals increase in fat replacement in skeletal muscles. J Cachexia Sarcopenia Muscle. https://doi.org/10.1002/jcsm.12555
Morrow JM, Sinclair CDJ, Fischmann A et al (2016) MRI biomarker assessment of neuromuscular disease progression: a prospective observational cohort study. Lancet Neurol 15:65–77
Bachasson D, Reyngoudt H, Turk S, Benveniste O, Hogrel JY, Carlier PG (2017) Muscle alterations in sporadic inclusion body myositis assessed using quantitative nuclear magnetic resonance imaging and spectroscopy, ultrasound shear-wave elastography, and relationships with muscle function. Neuromuscul Disord 27:S123
Landon-Cardinal O, Koumako C, Hardouin G et al (2020) Severe axial and pelvifemoral muscle damage in immune-mediated necrotizing myopathy evaluated by whole-body MRI. Semin Arthritis Rheum https://doi.org/10.1016/j.semarthrit.2020.02.009
Wokke BH, van den Bergen JC, Hooijmans MT, Verschuuren JJ, Niks EH, Kan HE (2015) T2 relaxation times are increased in skeletal muscle of DMD but not BMD patients. Muscle Nerve 53:38–43
Hooijmans MT, Niks EH, Burakiewicz J, Verschuuren JJGM, Webb AG, Kan HE (2017) Elevated phosphodiester and T2 levels can be measured in the absence of fat infiltration in Duchenne muscular dystrophy patients. NMR Biomed 30:e3667
Mankodi A, Bishop CA, Auh S, Newbould RD, Fischbeck KH, Janiczek RL (2016) Quantifying disease activity in fatty-infiltrated skeletal muscle by IDEAL-CPMG in Duchenne muscular dystrophy. Neuromuscul Disord 26:650–658
Marty B, Coppa B, Carlier PG (2018) Monitoring skeletal muscle chronic fatty degenerations using fast NMR T1-mapping. Eur Radiol 28:4662–4668
Ricotti V, Evans MR, Sinclair CD et al (2016) Upper limb evaluation in Duchenne muscular dystrophy: fat-water quantification by MRI, muscle force and function define endpoints for clinical trials. PLoS One 11:e0162542
Gerhalter T, Gast LV, Marty B et al (2019) 23Na MRI depicts early changes in ion homeostasis in skeletal muscle tissue of patients with Duchenne muscular dystrophy. J Magn Reson Imaging 50:1103–1113
Naarding KJ, Reyngoudt H, van Zwet EW et al (2020) MRI vastus lateralis fat fraction predicts loss of ambulation in Duchenne muscular dystrophy. Neurology 94:e1386–e1394
Andersen G, Dahlqvist JR, Vissing CR, Heje K, Thomsen C, Vissing J (2017) MRI as outcome measure in facioscapulohumeral muscular dystrophy: 1-year follow-up of 45 patients. J Neurol 264:438–447
Arrigoni F, De Luca A, Velardo D et al (2018) Multiparametric quantitative MRI assessment of thigh muscles in limb-girdle muscular dystrophy 2A and 2B. Muscle Nerve 58:550–558
Fischmann A, Hafner P, Gloor M et al (2013) Quantitative MRI and loss of free ambulation in Duchenne muscular dystrophy. J Neurol 260:969–974
Fischer D, Hafner P, Rubino D et al (2016) The 6-minute walk test, motor function measure and quantitative thigh muscle MRI in Becker muscular dystrophy: a cross-sectional study. Neuromuscul Disord 26:414–422
Yao L, Yip AL, Shrader JA et al (2016) Magnetic resonance measurement of muscle T2, fat-corrected T2 and fat fraction in the assessment of idiopathic inflammatory myopathies. Rheumatology (Oxford) 55:441–449
Cohen J (1988) Statistical power analysis for the behavioral sciences. L. Erlbaum Associates, Hillsdale
Middel B, Van Sonderen E (2002) Statistical significant change versus relevant or important change in (quasi) experimental design: some conceptual and methodological problems in estimating magnitude of intervention-related change in health services research. Int J Integr Care 2:e15
Azzabou N, de Sousa PL, Araujo EC, Carlier PG (2015) Validation of a generic approach to muscle water T2 determination at 3T in fat-infiltrated skeletal muscle. J Magn Reson Imaging 41:645–653
Chan BY, Gill KG, Rebsamen SL, Nguyen JC (2016) MR imaging of pediatric bone marrow. Radiographics 36:1911–1930
Hooijmans MT, Niks EH, Burakiewicz J (2017) Non-uniform muscle fat replacement along the proximodistal axis in Duchenne muscular dystrophy. Neuromuscul Disord 27:458–464
Reyngoudt H, Lopez Kolkovsky AL, Carlier PG (2019) Free intramuscular Mg2+ concentration calculated using both 31P and 1H NMRS-based pH in skeletal muscle of Duchenne muscular dystrophy patients. NMR Biomed 32:e4115
Willcocks RJ, Rooney WD, Triplett WT et al (2016) Multicenter prospective longitudinal study of magnetic resonance biomarkers in a large Duchenne muscular dystrophy cohort. Ann Neurol 79:535–547
Addison O, Marcus RL, Lastayo PC, Ryan AS (2014) Intermuscular fat: a review of the consequences and causes. Int J Endocrinol 2014:1–11
Pons C, Borotikar B, Garetier M et al (2018) Quantifying skeletal muscle volume and shape in humans using MRI: a systematic review of validity and reliability. PLoS One 13:1–26
Carlier PG, Shukelovich A, Baudin P-Y, Boisserie J-M, Le Louër J, Azzabou N (2014) Fast, precise, interactive segmentation of skeletal muscle NMR images. Neuromuscul Disord 24:836–837
Baudin P-Y, Beyeler M, Carlier PG, Scheidegger O (2017) Interactive segmentation of leg muscles in NMR images. J Neuromuscul Dis 27:S126
Snezhko E, Azzabou N, Baudin PY, Carlier PG (2018) Convolutional neural network segmentation of skeletal muscle NMR images Proc 27th ISMRM 2800
Ghosh S, Ray N, Boulanger P (2017) A structured deep-learning based approach for the automated segmentation of human leg muscle from 3D MRI. Proc IEEE Int Conf Comput Vis 2017:117–123
Mesbah S, Shalaby AM, Stills S et al (2019) Novel stochastic framework for automatic segmentation of human thigh MRI volumes and its applications in spinal cord injured individuals. PLoS One 14:e0216487
Amer R, Nassar J, Bendahan D, Greenspan H, Ben-Eliezer N (2019) Automatic segmentation of muscle tissue and inter-muscular fat in thigh and calf MRI images. Med Image Comput Comput Assist Interv 11765:219–227
Li H, Luo H, Liu Y (2020) Paraspinal muscle segmentation based on deep neural network. Sensors (Basel) 19:e2650
Verdu-Diaz J, Alonso-Perez J, Nunez-Peralta C et al (2020) Accuracy of a machine learning muscle MRI-based tool for the diagnosis of muscular dystrophies. Neurology 94:e1094–e1102
Acknowledgments
The authors thank the patients, their families and caregivers, for their participation in the respective studies. Also, the assistance of many people involved in this work, including Mélanie Annoussamy and colleagues from I-Motion (Trousseau Hospital, Paris, France), Jean-Yves Hogrel from the Neuromuscular Investigation Center at the Institute of Myology (Paris, France), as well as the Neurology and MRI departments at Cincinnati Children’s Hospital Medical Center (Cincinnati, Ohio, USA) is greatly acknowledged. Some of the data presented in this work were collected as part of the BIOIMAGE-NMD FP7 framework program for research, technological development and demonstration (DMD data from Cincinnati), the AFM-funded DMD natural history study, the Clinical Outcome Study for Dysferlinopathy steered by the JAIN foundation, and the SMA natural history study co-funded by Roche and the Institute of Myology.
Funding
The PRO-DMD-01 natural history study (NCT01753804) was sponsored by Prosensa Therapeutics B.V. and BioMarin. The DMD natural history study (NCT02780492) was sponsored by AFM. The DYSF COS natural history study (NCT01676077) was sponsored by the JAIN foundation. The SMA natural history study (NCT0239183) was sponsored by Roche and the Institute of Myology. The EU Bioimage-NMD program with project ID 602485 was funded under FP7-HEALTH-2013-INNOVATION-1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Harmen Reyngoudt, PhD.
Conflict of interest
HR, BM, JMB, JLL, CK, PYB, TS, AB, TG, YA, OB and PGC report no disclosures relevant to the manuscript; BW worked as an investigator of clinical trials of BioMarin (including the PRO-DMD-01 NCT01753804 natural history study that included the DMD cohorts in this study) and received honoraria from serving on the advisory board of BioMarin; LS worked as an investigator for the NCT02780492 DMD natural history study and the NCT0239183 SMA natural history study co-funded by Roche and he performed consultancy for Roche, Avexis, Cytokinetics, Biogen, Sarepta, Biomarin, Pfizer, Biophytis.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in
Gidaro T, Reyngoudt H, Le Louër J et al (2020) Quantitative nuclear magnetic resonance imaging detects subclinical changes over 1 year in skeletal muscle of GNE myopathy. J Neurol 267:228-238.
Willis TA, Hollingsworth KG, Coombs A et al (2013) Quantitative muscle MRI as an assessment tool for monitoring disease progression in LGMD2I: A multicentre longitudinal study. PLoS One 8:e70993.
Carlier PG, Azzabou N, de Sousa PL et al (2015) Skeletal muscle quantitative nuclear magnetic resonance imaging follow-up of adult Pompe patients. J Inherit Metab Dis 38:565–572.
Naarding KJ, Reyngoudt H, van Zwet EW et al (2020) MRI vastus lateralis fat fraction predicts loss of ambulation in Duchenne muscular dystrophy. Neurology 94:e1386-1394.
Chabanon A, Seferian AM, Daron A et al (2018) Prospective and longitudinal natural history study of patients with Type 2 and 3 spinal muscular atrophy: Baseline data NatHis-SMA study. PLoS One 13:e0201004.
Methodology
• retrospective
• observational
• multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Reyngoudt, H., Marty, B., Boisserie, JM. et al. Global versus individual muscle segmentation to assess quantitative MRI-based fat fraction changes in neuromuscular diseases. Eur Radiol 31, 4264–4276 (2021). https://doi.org/10.1007/s00330-020-07487-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07487-0