Abstract
Objectives
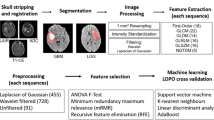
Deep learning-based automatic segmentation (DLAS) helps the reproducibility of radiomics features, but its effect on radiomics modeling is unknown. We therefore evaluated whether DLAS can robustly extract anatomical and physiological MRI features, thereby assisting in the accurate assessment of treatment response in glioblastoma patients.
Methods
A DLAS model was trained on 238 glioblastomas and validated on an independent set of 98 pre- and 86 post-treatment glioblastomas from two tertiary hospitals. A total of 1618 radiomics features from contrast-enhanced T1-weighted images (CE-T1w) and histogram features from apparent diffusion coefficient (ADC) and cerebral blood volume (CBV) mapping were extracted. The diagnostic performance of radiomics features and ADC and CBV parameters for identifying treatment response was tested using area under the curve (AUC) from receiver operating characteristics analysis. Feature reproducibility was tested using a 0.80 cutoff for concordance correlation coefficients.
Results
Reproducibility was excellent for ADC and CBV features (ICC, 0.82–0.99) and first-order features (pre- and post-treatment, 100% and 94.1% remained), but lower for texture (79.0% and 69.1% remained) and wavelet-transformed (81.8% and 74.9% remained) features of CE-T1w. DLAS-based radiomics showed similar performance to human-performed segmentations in internal validation (AUC, 0.81 [95% CI, 0.64–0.99] vs. AUC, 0.81 [0.60–1.00], p = 0.80), but slightly lower performance in external validation (AUC, 0.78 [0.61–0.95] vs. AUC, 0.65 [0.46–0.84], p = 0.23).
Conclusion
DLAS-based feature extraction showed high reproducibility for first-order features from anatomical and physiological MRI, and comparable diagnostic performance to human manual segmentations in the identification of pseudoprogression, supporting the utility of DLAS in quantitative MRI analysis.
Key Points
• Deep learning-based automatic segmentation (DLAS) enables fast and robust feature extraction from diffusion- and perfusion-weighted MRI.
• DLAS showed high reproducibility in first-order feature extraction from anatomical, diffusion, and perfusion MRI across two centers.
• DLAS-based radiomics features showed comparable diagnostic accuracy to manual segmentations in post-treatment glioblastoma.




Similar content being viewed by others
Abbreviations
- 3D:
-
Three-dimensional
- ADC:
-
Apparent diffusion coefficient
- AUC:
-
Area under the curve
- CBV:
-
Cerebral blood volume
- CCC:
-
Concordance correlation coefficient
- CE-T1w:
-
Contrast-enhanced T1-weighted
- DICE:
-
Dice similarity coefficient
- DLAS:
-
Deep learning-based automatic segmentation
- TP:
-
Tumor progression
References
Wen PY, Chang SM, Van den Bent MJ, Vogelbaum MA, Macdonald DR, Lee EQ (2017) Response assessment in neuro-oncology clinical trials. J Clin Oncol 35:2439–2449
Kickingereder P, Isensee F, Tursunova I et al (2019) Automated quantitative tumour response assessment of MRI in neuro-oncology with artificial neural networks: a multicentre, retrospective study. Lancet Oncol 20:728–740
Parmar C, Velazquez ER, Leijenaar R et al (2014) Robust radiomics feature quantification using semiautomatic volumetric segmentation. Plos One 9
Pavic M, Bogowicz M, Wurms X et al (2018) Influence of inter-observer delineation variability on radiomics stability in different tumor sites. Acta Oncol 57:1070–1074
Kumar V, Gu YH, Basu S et al (2012) Radiomics: the process and the challenges. Magn Reson Imaging 30:1234–1248
Lambin P, Leijenaar RTH, Deist TM et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14:749–762
Lambin P, Rios-Velazquez E, Leijenaar R et al (2012) Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 48:441–446
Park JE, Kim HS (2018) Radiomics as a quantitative imaging biomarker: practical considerations and the current standpoint in neuro-oncologic studies. Nucl Med Mol Imaging 52:99–108
Menze BH, Jakab A, Bauer S et al (2015) The multimodal brain tumor image segmentation benchmark (BRATS). IEEE Trans Med Imaging 34:1993–2024
Ellingson BM, Cloughesy TF, Zaw T et al (2012) Functional diffusion maps (fDMs) evaluated before and after radiochemotherapy predict progression-free and overall survival in newly diagnosed glioblastoma. Neuro Oncol 14:333–343
Law M, Young RJ, Babb JS et al (2008) Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 247:490–498
Kickingereder P, Burth S, Wick A et al (2016) Radiomic profiling of glioblastoma: identifying an imaging predictor of patient survival with improved performance over established clinical and radiologic risk models. Radiology 280:880–889
Bae S, Choi YS, Ahn SS et al (2018) Radiomic MRI phenotyping of glioblastoma: improving survival prediction. Radiology 289:797–806
Kim JY, Park JE, Jo Y et al (2018) Incorporating diffusion- and perfusion-weighted MRI into a radiomics model improves diagnostic performance for pseudoprogression in glioblastoma patients. Neuro Oncol. https://doi.org/10.1093/neuonc/noy133
Kickingereder P, Gotz M, Muschelli J et al (2016) Large-scale radiomic profiling of recurrent glioblastoma identifies an imaging predictor for stratifying anti-angiogenic treatment response. Clin Cancer Res 22:5765–5771
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820
Nolden M, Zelzer S, Seitel A et al (2013) The medical imaging interaction toolkit: challenges and advances : 10 years of open-source development. Int J Comput Assist Radiol Surg 8:607–620
Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC (2011) A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54:2033–2044
Shinohara RT, Sweeney EM, Goldsmith J et al (2014) Statistical normalization techniques for magnetic resonance imaging. Neuroimage Clin 6:9–19
Kang D, Park JE, Kim YH et al (2018) Diffusion radiomics as a diagnostic model for atypical manifestation of primary central nervous system lymphoma: development and multicenter external validation. Neuro Oncol. https://doi.org/10.1093/neuonc/noy021
Zwanenburg A, Leger S, Vallières M, Löck S (2016) Image biomarker standardisation initiative. arXiv preprint arXiv:1612.07003
Weisskoff RM, Boxerman JL, Sorensen AG et al (1994) Simultaneous blood volume and permeability mapping using a single Gd-based contrast injection. In: Proceedings of the Society of Magnetic Resonance, Second Annual Meeting; 1994 Aug 6–12; San Francisco, Calif.; Berkeley, Calif: Society of Magnetic Resonance, 279
Chung WJ, Kim HS, Kim N, Choi CG, Kim SJ (2013) Recurrent glioblastoma: optimum area under the curve method derived from dynamic contrast-enhanced T1-weighted perfusion MR imaging. Radiology 269:561–568
Kim HS, Suh CH, Kim N, Choi CG, Kim SJ (2014) Histogram analysis of intravoxel incoherent motion for differentiating recurrent tumor from treatment effect in patients with glioblastoma: initial clinical experience. AJNR Am J Neuroradiol 35:490–497
Dice LR (1945) Measures of the amount of ecologic association between species. Ecology 26:297–302
Kursa MB (2014) Robustness of Random Forest-based gene selection methods. BMC Bioinformatics 15(1):8
Loh WY (2011) Classification and regression trees. Wiley Interdisciplinary Reviews: Data Min Knowl Discov 1(1):14-23
Lin L, Dou Q, Jin YM et al (2019) Deep learning for automated contouring of primary tumor volumes by MRI for nasopharyngeal carcinoma. Radiology 291:677–686
Norman B, Pedoia V, Majumdar S (2018) Use of 2D U-Net convolutional neural networks for automated cartilage and meniscus segmentation of knee MR imaging data to determine relaxometry and morphometry. Radiology 288:177–185
Barboriak DP, Zhang Z, Desai P et al (2019) Interreader variability of dynamic contrast-enhanced MRI of recurrent glioblastoma: the multicenter ACRIN 6677/RTOG 0625 study. Radiology 290:467–476
Newitt DC, Zhang Z, Gibbs JE et al (2019) Test-retest repeatability and reproducibility of ADC measures by breast DWI: results from the ACRIN 6698 trial. J Magn Reson Imaging 49:1617–1628
Ellingson BM, Bendszus M, Boxerman J et al (2015) Consensus recommendations for a standardized brain tumor imaging protocol in clinical trials. Neuro Oncol 17:1188–1198
Baessler B, Weiss K, Pinto Dos Santos D (2019) Robustness and reproducibility of radiomics in magnetic resonance imaging: a phantom study. Invest Radiol 54:221–228
Milletari F, Navab N, Ahmadi S-A (2016) V-net: fully convolutional neural networks for volumetric medical image segmentation. In 2016 fourth international conference on 3D vision (3DV) (pp. 565-571). IEEE
Estienne T, Lerousseau M, Vakalopoulou M et al (2020) Deep learning-based concurrent brain registration and tumor segmentation. Front Comput Neurosci 14:17
Zwanenburg A, Vallieres M, Abdalah MA et al (2020) The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology 295:328–338
Zhao B, Tan Y, Tsai WY et al (2016) Reproducibility of radiomics for deciphering tumor phenotype with imaging. Sci Rep 6:23428
Park CM (2019) Can artificial intelligence fix the reproducibility problem of radiomics? Radiology. https://doi.org/10.1148/radiol.2019191154:191154
Funding
This research was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (grant number: NRF-2020R1A2B5B01001707 and NRF-2020R1A2C4001748).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Namkug Kim.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise (Seo Young Park, 8 years of experienced statistician).
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
The external 33 post-treatment glioblastoma patients were in part of a previously reported study (Kim JY et al Neuro-Oncology, Volume 21, Issue 3, March 2019, Pages 404–414, https://doi.org/10.1093/neuonc/noy133). This prior article dealt with development of multiparametric MRI radiomics model using manual segmentation whereas in this manuscript we report reproducibility and accuracy from DLAS-obtained radiomics features and focused on CE-T1w imaging. The method is totally different that the previous study used feature selection in order to create the model and only 12 features were used. Meanwhile what we did here was using entire 1618 extracted features and used random forest classifier to calculate diagnostic performance, with the aim to measure the effect of segmentation to subsequent feature extraction. Also, we created the DLAS model in this study while previous report did manual segmentation.
Methodology
• retrospective
• cross-sectional study
• multicentre study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 340 kb)
Rights and permissions
About this article
Cite this article
Park, J.E., Ham, S., Kim, H.S. et al. Diffusion and perfusion MRI radiomics obtained from deep learning segmentation provides reproducible and comparable diagnostic model to human in post-treatment glioblastoma. Eur Radiol 31, 3127–3137 (2021). https://doi.org/10.1007/s00330-020-07414-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07414-3