Abstract
Objectives
To assess how modifying multiple protocol parameters affects the dose and diagnostic performance of a neck CT protocol using patient-mimicking phantoms and task-based methods.
Methods
Six patient-mimicking neck phantoms containing hypodense lesions of 1 cm diameter and 30 HU contrast and one non-lesion phantom were examined with 36 CT protocols. All possible combinations of the following parameters were investigated: 100- and 120-kVp tube voltage; tube current modulation (TCM) noise levels of SD 7.5, 10, and 14; pitches of 0.637, 0.813, and 1.388; filtered back projection (FBP); and iterative reconstruction (AIDR 3D). Dose-length products (DLPs) and lesion detectability (assessed by 14 radiologists) were compared with the clinical standard protocol (120 kVp, TCM SD 7.5, 0.813 pitch, AIDR 3D).
Results
The DLP of the standard protocol was 25 mGy•cm; the area under the curve (AUC) was 0.839 (95%CI: 0.790–0.888). Combined effects of tube voltage reduction to 100 kVp and TCM noise level increase to SD 10 optimized protocol performance by improving dose (7.3 mGy•cm) and detectability (AUC 0.884, 95%CI: 0.844–0.924). Diagnostic performance was significantly affected by the TCM noise level at 120 kVp (AUC 0.821 at TCM SD 7.5 vs. 0.776 at TCM SD 14, p = 0.003), but not at 100-kVp tube voltage (AUC 0.839 at TCM SD 7.5 vs. 0.819 at TCM SD 14, p = 0.354), the reconstruction method at 100 kVp (AUC 0.854 for AIDR 3D vs. 0.806 for FBP, p < 0.001), but not at 120-kVp tube voltage (AUC 0.795 for AIDR 3D vs. 0.793 for FBP, p = 0.822), and the tube voltage for AIDR 3D reconstruction (p < 0.001), but not for FBP (p = 0.226).
Conclusions
Combined effects of 100-kVp tube voltage, TCM noise level of SD 10, a pitch of 0.813, and AIDR 3D resulted in an optimal neck protocol in terms of dose and diagnostic performance. Protocol parameters were subject to complex interactions, which created opportunities for protocol improvement.
Key Points
• A task-based approach using patient-mimicking phantoms was employed to optimize a CT system for neck imaging through systematic testing of protocol parameters.
• Combined effects of 100-kVp tube voltage, TCM noise level of SD 10, a pitch of 0.813, and AIDR 3D reconstruction resulted in an optimal protocol in terms of dose and diagnostic performance.
• Interactions of protocol parameters affect diagnostic performance and should be considered when optimizing CT techniques.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Computed tomography (CT) acquisition protocols should provide diagnostic image quality at the lowest possible dose in line with the ALARA principle (as low as reasonably achievable). Protocol settings used in clinical routine determine diagnostic reliability and dose exposure of patients [1]. On a larger scale, they affect the overall radiation burden to the population from CT examinations [2]. Previous work has shown that CT acquisition protocols and related dose exposure vary substantially across facilities [3, 4]. Protocol optimization thus offers significant potential for improving patient safety.
Prior efforts to optimize protocols harmonized dose exposure across CT facilities through protocol revision by expert panels and best practice sharing [5, 6]. Such programs were shown to be valuable in reducing dose and identifying dose outliers. However, they were not intended to assess diagnostic image quality, which means that they could neither exclude unacceptable image quality nor identify optimal protocols that strike the best balance between dose and image quality. Protocol optimization for such purposes can be defined in terms of determining how to better use a system’s imaging capabilities for obtaining adequate diagnostic information at the lowest possible dose.
Today, CT protocols routinely involve technologies that reduce dose, but also have complex effects on image properties. Especially the use of iterative reconstruction algorithms is associated with complex interactions between noise, texture, contrast, and spatial resolution [7,8,9], which are not adequately assessed with traditional metrics such as contrast-to-noise ratios [10]. In light of this situation, methods are of interest that reliably predict clinical image performance and allow comparison of protocols independently of the CT techniques involved. The recent report of the AAPM task group 233 on CT performance evaluation has highlighted the role of task-based methods for such purposes [11].
Task-based methods assess image quality by testing how well CT images enable an observer to perform detection tasks that are similar to clinical diagnostic tasks of radiologists [12]. The simplest of such tasks is the detection of a signal (e.g., a low-contrast lesion) against a uniform phantom background [13]. However, patients are not uniform, and the complexity of background texture affects image properties and detection outcomes [7, 14]. A further development towards assessing CT protocols more realistically would therefore involve quantitative assessment of detection outcomes in anatomically more realistic phantoms.
Recent work has introduced 3D-printed phantoms that realistically mimic a patient’s contrast medium–enhanced neck and contain low-contrast lesions for task-based image quality assessment [15]. The present study builds on these methods and uses patient-mimicking phantoms with embedded low-contrast lesions to assess protocol parameters in neck CT imaging. The hypothesis was that, through the testing of protocol parameters in a realistic setting, optimized parameter combinations that make better use of the imaging techniques available on a specific CT system can be found to ensure diagnostic image quality at lower dose for a given application. Based on these assumptions, the aim was to assess how modifying multiple protocol parameters affects the dose and diagnostic performance of a neck CT protocol using patient-mimicking phantoms and task-based methods.
Methods
Study design
The institutional ethics committee approved the study and waived informed consent. Ethics approval was obtained to perform the study with seven 3D printed phantoms mimicking a patient’s neck (six phantoms containing a low-contrast lesion, one non-lesion phantom). The phantoms were examined with 36 different CT protocols. Image quality was assessed by 14 radiologists using task-based methods (8064 readings in total). The protocols were analyzed for dose and image quality and compared with our clinical standard protocol.
Phantoms
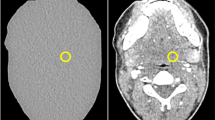
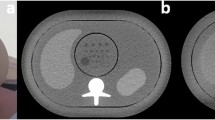
Seven anatomically identical phantoms mimicking a patient’s neck were created based on previously published methods [15]. Briefly, the phantoms were produced from seven different versions of a CT image of a patient’s neck: the original, unmodified CT image and six versions of the same image, where lesions of 1 cm diameter were inserted through pixelwise subtraction of 30 HU in a circular region of interest in different locations throughout the parapharyngeal space. Radiopaque 3D printing with potassium-iodide-doped ink and paper-based 3D printing were used to produce the phantoms with 1 cm thickness [16, 17]. The method of phantom creation was the same as described in more detail previously [15], except that all lesions had 30 HU contrast and were distributed throughout the parapharyngeal space. This lesion contrast was selected to create lesions at the interface between detectable and undetectable based on that previous study. Six phantoms thus contained one low-contrast lesion of 1 cm diameter and 30 HU contrast in different locations of the parapharyngeal space. One phantom did not contain any lesion. Figure 1 shows illustrations and CT images of the phantoms. For image acquisition, the 1-cm-thick phantoms were inserted into a full-size head and neck phantom as shown in suppl. fig. 1.
Drawings and CT images of the phantoms used for protocol assessment. Lesions are drawn in gray and indicated by white arrows in the CT images. The CT images shown here were acquired with 120-kVp tube voltage, TCM SD of 7.5, and a pitch of 0.813 and reconstructed with AIDR 3D (corresponding to the reference protocol used in this study). All images are displayed with window level 40 and window width 350
CT acquisition
Images were acquired on a Canon Aquilion Prime CT scanner (Canon Medical Systems). All acquisitions covered 4 cm in z-direction, with the inserted phantoms in the center along the z-axis. The phantoms were imaged with a total of 36 different acquisition protocols to investigate all possible combinations of different tube voltage, tube current, pitch, and reconstruction settings (Fig. 2). For all acquisitions, 100-kVp tube voltage corresponded to the recommended setting of the automatic tube potential selection system. The tube current modulation (TCM) noise levels corresponded to the CT settings for high quality (SD 7.5), quality (SD 10), and standard (SD 14) as recommended by the CT vendor. Two acquisitions per protocol and phantom were performed. Images were reconstructed with 0.5-mm slice thickness and a soft tissue kernel (FC08). For analysis, the current clinical standard neck protocol was used as reference: 120-kVp, TCM SD of 7.5, 0.813 pitch, and AIDR 3D.
Dose assessment
Dose-length products (DLPs) were assessed. To account for the short scan length of 4 cm, the contribution of overscanning to the DLP was calculated using previously published methods [18] and reduced to 20%. The contribution of overscanning to the DLP was thus made equivalent to a scan coverage of 20 cm in z-direction.
Image quality assessment
Fourteen radiologists with prior training in neck CT imaging participated in the readings. Their experience ranged from 2 to 15 years (mean: 4.9, median: 4). Readers were presented with 16 images per acquisition protocol: one image per acquisition of the lesion phantoms and two images per acquisition of the non-lesion phantom. Two images of the non-lesion phantom were included to adjust the proportion between images showing lesions (for which six phantoms were used) and images not showing lesions (for which only one phantom was available). Each reader was thus presented with a total of 576 images (6 lesion phantoms × 2 acquisitions × 1 image per acquisition × 36 protocols + 1 non-lesion phantom × 2 acquisitions × 2 images per acquisition × 36 protocols). For each presented image, readers were asked to indicate whether a lesion was present. If a lesion was deemed present, they were asked to draw a region of interest containing the entire lesion. Readers were blinded to the experimental design in that they were unaware of how many different lesion positions were possible in the study setting. All readings were performed on diagnostic monitors (Eizo RadiForce RX250, Eizo Corporation) using in-house developed software.
Data analysis
The intersection over union (IOU) between the lesion ground truth and reader selection was calculated [19]. Reader responses were classified into the following: (1) no lesion marked, (2) lesion marked on negative samples, (3) IOU = 0, (4) IOU ≤ 0.5, and (5) IOU > 0.5. Receiver operating characteristic statistics were performed, and the area under the curve (AUC) was determined per reader and acquisition protocol. Based on these results, interrater reliability was determined using the intraclass correlation coefficient. AUC differences between each protocol and the reference protocol along with one-sided 95% confidence intervals (CI) were calculated. A non-inferiority analysis was performed to compare the AUC between each protocol and the reference protocol [20]. The limit of non-inferiority was set before the analysis and considered at 5% of the mean AUC value of the reference protocol (mean AUC 0.839, non-inferiority limit −0.042). Non-inferiority was assumed when the lower limit of the 95% CI (one-sided) was greater than the limit of non-inferiority. Superiority was assumed when the lower limit of the 95% CI (one-sided) was greater than 0. Inferiority was assumed when the upper limit of the 95% CI (one-sided) was less than 0. In addition, the effects of tube voltage, tube current, pitch, and image reconstruction parameters on AUC results were analyzed using a general linear model; p values for multiple comparisons were adjusted with Sidak’s method. Differences were interpreted as significant when p < 0.05.
Results
Dose and detectability results
The intraclass correlation coefficient between AUC results of the 14 participating radiologists was 0.73 (95% CI: 0.58 to 0.84). Reading time for all 576 images was approximately 90 min per participant. The reference protocol had a DLP of 25 mGy•cm with a mean AUC across all readers of 0.839 (95% CI: 0.790 to 0.888). Figure 3 shows the dose and detectability results for all protocols in relation to the reference protocol. Except for a pitch reduction to 0.637, all protocol modifications resulted in a lower dose. Suppl. Table 1 summarizes dose and detectability results, and Suppl. figure 2 provides a series of exemplary CT images acquired with the 36 protocols investigated.
Protocol improvement
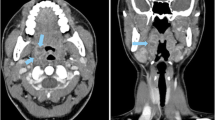
Figure 4 presents the results of the non-inferiority analysis. Compared with the reference protocol, seven protocols yielded non-inferior detectability at a reduced dose and thus improved protocol performance (Table 1). The strongest dose reduction at non-inferior detectability was achieved by reducing tube voltage to 100 kVp and increasing the TCM noise level to SD 14 (AUC 0.865, 95% CI: 0.824 to 0.905; DLP 5.1 mGy•cm). Two protocols reduced dose and yielded superior detection results, which means that dose exposure and image quality were improved simultaneously. One of these protocols used a lower tube voltage of 100 kVp and a higher pitch of 1.388, which increased the AUC to 0.891 (95% CI: 0.842 to 0.939) and reduced the DLP to 13.4 mGy•cm. The other protocol used a lower tube voltage of 100 kVp and higher TCM noise level of 10, which increased the AUC to 0.884 (95% CI: 0.844 to 0.924) and reduced the DLP even further to 7.3 mGy•cm. For comparison, Fig. 5 presents CT images acquired with these two protocols and the reference protocol.
Results of the non-inferiority analysis. The non-inferiority limit (indicated by grid lines) was set to −0.042, corresponding to 5% of the mean AUC value of the reference protocol (120-kVp tube voltage, TCM SD of 7.5, pitch of 0.813, AIDR 3D). Mean AUC differences between each protocol and the reference protocol along with one-sided 95% confidence intervals are indicated on top
Comparison of CT images acquired with the reference protocol and two protocols that yielded superior detectability results. a Reference protocol (120 kVp, TCM SD of 7.5, pitch of 0.813, AIDR 3D). b Reduced tube voltage and tube current (100 kVp, TCM SD of 10, pitch of 0.813, AIDR 3D). c Reduced tube voltage and increased pitch (100 kVp, TCM of 7.5, pitch of 1.388, AIDR 3D). The drawing indicates the lesion position, and lesions are additionally indicated by white arrows in the CT images. Images are displayed with window level/window width 40/350 at 120 kVp and 80/350 at 100 kVp
Protocol parameter effects
Figure 6 shows a comparison between a CT image acquired with the reference protocol and three CT images illustrating the effects of different parameter combinations: (1) acquired with reduced tube current (TCM SD 14) and reconstructed with AIDR 3D, (2) acquired with reduced tube voltage (100 kVp) and reduced tube current (TCM SD 14) and reconstructed with AIDR 3D, and (3) the same acquisition parameters as in (2) but reconstructed with FBP. A higher TCM noise level resulted in inferior detectability when combined with 120-kVp tube voltage while detectability was unchanged when combined with a reduced tube voltage of 100 kVp. Likewise, image reconstruction with FBP at reduced tube voltage and higher TCM noise level resulted in inferior detectability while reconstruction with AIDR 3D did not (Table 2).
CT images illustrating the effects of tube voltage reduction, tube current reduction, and the image reconstruction method used. a Reference protocol (120 kVp, TCM SD of 7.5, pitch of 0.813, AIDR 3D). b Reduced tube current (120 kVp, TCM SD of 14, pitch of 0.813, AIDR 3D). c Reduced tube voltage and tube current (100 kVp, TCM SD of 14, pitch of 0.813, AIDR 3D). d Same acquisition parameters as c but reconstructed with FBP instead of AIDR 3D. The drawing indicates the lesion position, and lesions are additionally indicated by white arrows in the CT images. Images are displayed with window level/window width 40/350 at 120 kVp and 80/350 at 100 kVp
These results were consistent with the results obtained with other parameter combinations. In a comparison across all protocols, a TCM noise level increase from 7.5 to 14 reduced detectability at 120-kVp tube voltage (AUC 0.821, 95% CI: 0.802 to 0.840 vs. 0.776, 95% CI: 0.757 to 0.795; p = 0.003). However, at 100 kVp, detectability was less markedly degraded by a TCM noise level increase from 7.5 to 14 (AUC 0.839, 95% CI: 0.820 to 0.858 vs. 0.819, 95% CI: 0.800 to 0.837; p = 0.354) and decreased only significantly in conjunction with a pitch of 1.388 (AUC 0.871, 95% CI: 0.839 to 0.904 vs. 0.789, 95% CI: 0.756 to 0.821; p = 0.001). Compared with FBP, AIDR 3D improved detectability at 100 kVp (AUC 0.806, 95% CI: 0.791 to 0.821 vs. 0.854, 95% CI: 0.838 to 0.869; p < 0.001), but not at 120-kVp tube voltage (AUC 0.793, 95% CI: 0.777 to 0.808 vs. 0.795, 95% CI: 0.780 to 0.810; p = 0.822). Conversely, lowering the tube voltage from 120 to 100 kVp significantly improved detectability with the use of AIDR 3D (p < 0.001), but not with FBP for image reconstruction (p = 0.226). These findings are summarized in Tables 3 and 4.
Discussion
CT protocol optimization has significant potential for improving patient safety by reducing dose exposure and/or enhancing the diagnostic yield of CT images. Optimizing protocols for clinical care requires the use of methods that are predictive of clinical performance. To this end, the present study assessed neck CT protocols using patient-mimicking phantoms and task-based methods. Thirty-six protocols were evaluated and compared with a clinical protocol combining 120-kVp tube voltage, a TCM noise level of 7.5, a pitch of 0.813, and image reconstruction with AIDR 3D. Protocol parameters were varied and their effect on dose exposure and detectability of low-contrast lesions in the parapharyngeal space was analyzed.
We identified seven protocols that reduced dose without yielding inferior detection results compared with the clinical reference protocol. The strongest dose reduction at non-inferior detectability was achieved with 100-kVp tube voltage, a TCM SD of 14, a pitch of 0.813, and AIDR 3D (DLP reduction from 25 to 5.1 mGy•cm). Two protocols achieved superior detectability, which means that diagnostic performance was improved while dose was reduced. Based on these results, an optimal protocol can be derived, which uses 100-kVp tube voltage, a TCM SD of 10, a pitch of 0.813, and AIDR 3D for image reconstruction. This protocol improves detectability while reducing the DLP from 25 to 7.3 mGy•cm compared with the reference protocol.
Each of the scan parameters varied in the present study individually affects dose and image quality. Lower tube voltages reduce dose while increasing noise and contrast [21] and have been reported previously to maintain or improve low-contrast detectability [22, 23]. Higher TCM noise levels reduce dose and increase noise and have been found to degrade low-contrast detectability in previous studies [24, 25]. Iterative reconstruction is noise- and contrast-dependent and affects noise, texture, and spatial resolution [8, 9]. Low-contrast detectability has been previously reported to improve with IR or to be equivalent to FBP [26, 27].
The results of the present study reflect how different combinations of these effects jointly affect a detection task in a clinical setting. For example, higher lesion contrast compensated for higher noise in most protocols with 100-kVp tube voltage, so that tube current could be reduced without compromising detectability. AIDR 3D reconstruction, which is noise- and contrast-dependent, enabled positive effects of tube voltage reduction on detectability, supporting previous reports of better lesion detection on 100-kVp IR than on 120-kVp FBP images [28]. Conversely, in our experiments, advantages of AIDR 3D over FBP were also more significant when a lower tube voltage (corresponding to greater noise and contrast) was used. The results illustrate the complexity that arises from varying multiple parameters and that conclusions regarding the advantages and disadvantages of particular CT techniques should consider the protocol context in which they were studied.
This complexity makes it desirable to compare different protocol scenarios directly and systematically and to predict their performance in clinical practice. The experimental study presented here therefore used task-based methods that have been developed for such purposes. However, our approach differed from most previous studies in that anatomical and not uniform phantoms were used, which is of relevance because texture and anatomic detail have been shown to affect image properties [7], human lesion perception [29], and the relationship between dose and image quality [30]. We therefore consider the search tasks used in the present study to be more complex and realistic, which should make the results more representative of clinical practice. A focus of future work will be to further develop and adapt the methodology for application to other anatomical regions and diagnostic tasks. Another focus will be to investigate whether the phantom design can be modified to facilitate image acquisition, e.g., by integrating multiple lesions simultaneously.
Limitations of the present study include that the results only apply to contrast medium–enhanced neck imaging and the CT scanner and techniques used in this study. For example, IR algorithms from different vendors have been shown to have different effects on low-contrast detectability [31, 32]. Also, due to the small number of phantoms investigated, signal locations were not completely random. However, the risk of bias was reduced as readers were unaware of the number of phantoms and signal locations. Detectability was assessed by human observers, which is most representative of the performance of radiologists in the clinical setting but also subject to significant variability and time-consuming. Future work could address this limitation by using a model observer approach [33].
CT protocols vary considerably between scanners and institutions and it is likely that many patients could be examined more efficiently. Our study presents an approach to test and optimize protocol parameters in a realistic context in order to use the imaging techniques of a CT system more efficiently to deliver diagnostic information. The results illustrate how interactions between protocol parameters affect diagnostic performance, which should be borne in mind when assessing the diagnostic effects of CT techniques.
Abbreviations
- AIDR 3D:
-
Adaptive iterative dose reduction 3D
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- DLP:
-
Dose-length product
- FBP:
-
Filtered back projection
- HU:
-
Hounsfield unit
- IOU:
-
Intersection over union
- IR:
-
Iterative reconstruction
- SD:
-
Standard deviation
- TCM:
-
Tube current modulation
References
Rehani MM, Yang K, Melick ER et al (2020) Patients undergoing recurrent CT scans: assessing the magnitude. Eur Radiol 30:1828–1836
Smith-Bindman R, Miglioretti DL, Johnson E et al (2012) Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996-2010. JAMA 307:2400–2409
Smith-Bindman R, Wang Y, Yellen-Nelson TR et al (2017) Predictors of CT radiation dose and their effect on patient care: a comprehensive analysis using automated data. Radiology 282:182–193
Racine D, Ryckx N, Ba A et al (2018) Task-based quantification of image quality using a model observer in abdominal CT: a multicentre study. Eur Radiol 28:5203–5210
Goenka AH, Dong F, Wildman B, Hulme K, Johnson P, Herts BR (2015) CT radiation dose optimization and tracking program at a large quaternary-care health care system. J Am Coll Radiol 12:703–710
Demb J, Chu P, Nelson T et al (2017) Optimizing radiation doses for computed tomography across institutions: dose auditing and best practices. JAMA Intern Med 177:810–817
Solomon J, Samei E (2014) Quantum noise properties of CT images with anatomical textured backgrounds across reconstruction algorithms: FBP and SAFIRE. Med Phys 41:091908
Richard S, Husarik DB, Yadava G, Murphy SN, Samei E (2012) Towards task-based assessment of CT performance: system and object MTF across different reconstruction algorithms. Med Phys 39:4115–4122
Yu L, Vrieze TJ, Leng S, Fletcher JG, McCollough CH (2015) Technical note: measuring contrast- and noise-dependent spatial resolution of an iterative reconstruction method in CT using ensemble averaging. Med Phys 42:2261–2267
Christianson O, Chen JJ, Yang Z et al (2015) An improved index of image quality for task-based performance of CT iterative reconstruction across three commercial implementations. Radiology 275:725–734
Samei E, Bakalyar D, Boedeker KL et al (2019) Performance evaluation of computed tomography systems: summary of AAPM task group 233. Med Phys 46:e735–e756
Barrett HH, Myers KJ, Hoeschen C, Kupinski MA, Little MP (2015) Task-based measures of image quality and their relation to radiation dose and patient risk. Phys Med Biol 60:R1–R75
Racine D, Viry A, Becce F et al (2017) Objective comparison of high-contrast spatial resolution and low-contrast detectability for various clinical protocols on multiple CT scanners. Med Phys 44:e153–e163
Solomon J, Ba A, Bochud F, Samei E (2016) Comparison of low-contrast detectability between two CT reconstruction algorithms using voxel-based 3D printed textured phantoms. Med Phys 43:6497
Ardila Pardo GL, Conzelmann J, Genske U, Hamm B, Scheel M, Jahnke P (2020) 3D printing of anatomically realistic phantoms with detection tasks to assess the diagnostic performance of CT images. Eur Radiol 30:4557–4563
Jahnke P, Limberg FR, Gerbl A et al (2017) Radiopaque three-dimensional printing: a method to create realistic CT phantoms. Radiology 282:569–575
Jahnke P, Schwarz S, Ziegert M, Schwarz FB, Hamm B, Scheel M (2019) Paper-based 3D printing of anthropomorphic CT phantoms: Feasibility of two construction techniques. Eur Radiol 29:1384–1390
Tsalafoutas IA, Metallidis SI (2011) A method for calculating the dose length product from CT DICOM images. Br J Radiol 84:236–243
Jaccard P (1912) The distribution of the flora in the alpine zone. New Phytol 11:37–50
Evaluation of Medicines for Human Use. Points to consider on switching between superiority and non-inferiority (CPMP/ EWP/482/99). The European Agency for the Evaluation of Medicinal Products http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guide-line/2009/09/WC500003658.pdf. Accessed January 15, 2020
Kalva SP, Sahani DV, Hahn PF, Saini S (2006) Using the K-edge to improve contrast conspicuity and to lower radiation dose with a 16-MDCT: a phantom and human study. J Comput Assist Tomogr 30:391–397
Brinkley MF, Ramirez-Giraldo JC, Samei E et al (2016) Effects of automatic tube potential selection on radiation dose index, image quality, and lesion detectability in pediatric abdominopelvic CT and CTA: a phantom study. Eur Radiol 26:157–166
Euler A, Stieltjes B, Szucs-Farkas Z et al (2017) Impact of model-based iterative reconstruction on low-contrast lesion detection and image quality in abdominal CT: a 12-reader-based comparative phantom study with filtered back projection at different tube voltages. Eur Radiol 27:5252–5259
Kanal KM, Chung JH, Wang J et al (2011) Image noise and liver lesion detection with MDCT: a phantom study. AJR Am J Roentgenol 197:437–441
McCollough CH, Yu L, Kofler JM et al (2015) Degradation of CT low-contrast spatial resolution due to the use of iterative reconstruction and reduced dose levels. Radiology 276:499–506
Joemai RM, Veldkamp WJ, Kroft LJ, Hernandez-Giron I, Geleijns J (2013) Adaptive iterative dose reduction 3D versus filtered back projection in CT: evaluation of image quality. AJR Am J Roentgenol 201:1291–1297
Schindera ST, Odedra D, Raza SA et al (2013) Iterative reconstruction algorithm for CT: can radiation dose be decreased while low-contrast detectability is preserved? Radiology 269:511–518
Husarik DB, Schindera ST, Morsbach F et al (2014) Combining automated attenuation-based tube voltage selection and iterative reconstruction: a liver phantom study. Eur Radiol 24:657–667
Samei E, Flynn MJ, Eyler WR (1999) Detection of subtle lung nodules: relative influence of quantum and anatomic noise on chest radiographs. Radiology 213:727–734
Kotre CJ (1998) The effect of background structure on the detection of low contrast objects in mammography. Br J Radiol 71:1162–1167
Jensen K, Martinsen AC, Tingberg A, Aalokken TM, Fosse E (2014) Comparing five different iterative reconstruction algorithms for computed tomography in an ROC study. Eur Radiol 24:2989–3002
Greffier J, Frandon J, Larbi A, Beregi JP, Pereira F (2020) CT iterative reconstruction algorithms: a task-based image quality assessment. Eur Radiol 30:487–500
Gong H, Yu L, Leng S et al (2019) A deep learning- and partial least square regression-based model observer for a low-contrast lesion detection task in CT. Med Phys 46:2052–2063
Acknowledgments
We thank our colleagues from the Department of Radiology and Neuroradiology for participating in the detectability experiment, Arthur Emig from the Department of Radiology and Pimrapat Gebert from the Institute of Biometry and Clinical Epidemiology for support with statistical analysis, and Bettina Herwig for assistance with the preparation of the article.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study has received funding by the Bundesministerium für Wirtschaft und Energie (DE): 03EFHBE093.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Paul Jahnke.
Conflict of interest
Dr. Jahnke and PD Dr. Scheel are shareholders of a company manufacturing CT phantoms.
Statistics and biometry
Pimrapat Gebert kindly provided statistical advice for this manuscript.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1.32 mb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jahnke, P., Conzelmann, J., Genske, U. et al. Task-based assessment of neck CT protocols using patient-mimicking phantoms—effects of protocol parameters on dose and diagnostic performance. Eur Radiol 31, 3177–3186 (2021). https://doi.org/10.1007/s00330-020-07374-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07374-8