Abstract
Objective
To determine CT’s role in the early detection of COVID-19 infection and serial CT changes in the disease course in patients with COVID-19 pneumonia.
Methods
From January 21 to February 18, 2020, all of the patients who were suspected of novel coronavirus infection and verified by RT-PCR tests were retrospectively enrolled in our study. All of the patients underwent serial RT-PCR tests and serial CT imaging. The temporal relationship between the serial RT-PCR results (negative conversion to positive, positive to negative) and serial CT imaging was investigated, and serial CT changes were evaluated.
Results
A total of 155 patients with confirmed COVID-19 pneumonia were evaluated. Chest CT detection time of COVID-19 pneumonia was 2.61 days earlier than RT-PCR test (p = 0.000). The lung CT improvement time was significantly shorter than that of RT-PCR conversion to negative (p = 0.000). Three stages were identified from the onset of the initial symptoms: stage 1 (0–3 days), stage 2 (4–7 days), and stage 3 (8–14 days and later). Ground glass opacity (GGO) was predominant in stage 1, then consolidation and crazy paving signs were dramatically increased in stage 2. In stage 3, fibrotic lesions were rapidly increased. There were significant differences in the main CT features (p = 0.000), number of lobes involved (p = 0.001), and lesion distribution (p = 0.000) among the different stages.
Conclusion
Chest CT detected COVID-19 pneumonia earlier than the RT-PCR results and can be used to monitor disease course. Combining imaging features with epidemiology history and clinical information could facilitate the early diagnosis of COVID-19 pneumonia.
Key Points
• The chest CT detection time of COVID-19 pneumonia was 2.61 days earlier than that of an initial RT-PCR positive result (t = − 7.31, p = 0.000).
• The lung CT improvement time was significantly shorter than that of RT-PCR conversion to negative (t = − 4.72, p = 0.000).
• At the early stage (0–3 days), the CT features of COVID-19 were predominantly GGO and small-vessel thickening; at stage 2 (4–7 days), GGO evolved to consolidation and crazy paving signs. At stage 3 (8–14 days and later), fibrotic lesions significantly increased, accompanied by consolidation, GGO, and crazy paving signs.
Similar content being viewed by others
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported in Wuhan, Hubei Province, China, in December 2019 [1]. In the following weeks, infections spread rapidly across China and other countries [2]. Published studies showed that COVID-19 had the feature of strong human-to-human transmission [2, 3]. The Chinese government implemented strict quarantine measures to contain the spread of the disease with obvious effects. However, COVID-19 rapidly spread worldwide. On March 27, 2020, the WHO reported more than half a million confirmed cases of COVID-19 and more than 20,000 deaths [4].
To control the spread of the disease, early identification of COVID-19 infection and immediate isolation of patients are essential. Real-time reverse transcription polymerase chain reaction (RT-PCR) was the reference standard for a definite diagnosis of COVID-19 infection according to the guideline of the Diagnosis and Treatment of Pneumonia Caused by COVID-19 (Trial Version 3) published by the National Health Commission of the People’s Republic of China (NHCPRC) [5], which is the key indicator for hospitalization. However, the low sensitivity of RT-PCR (71%) [6] and lack of RT-PCR assays in the early stage of the outbreak limited the prompt diagnosis of infected patients, which means that many COVID-19 patients were not detected and timely isolated. These patients were bound to become infection sources and infect a large population due to the highly contagious nature of the virus. The reasons for the low efficiency of viral nucleic acid detection included (1) immature development of nucleic acid detection technology, (2) variations in the detection rates from different manufacturers, (3) low patient viral loads, and (4) improper clinical sampling [6].
Chest computed tomography (CT) is a non-invasive diagnostic imaging tool for pneumonia that is relatively easy to perform and provides rapid diagnoses. The sensitivity of chest CT in the detection of COVID-19 pneumonia was 98% [6]. Studies have reported the imaging features, including GGO (ground glass opacity), patchy consolidation, and crazy paving signs [7,8,9]. However, few studies reported the time differences in RT-PCR results and CT abnormalities [6, 10], or focused on the dynamic CT changes in COVID-19 pneumonia [11]. To better understand the early diagnostic value of chest CT and the disease course, we investigated the temporal relationship between serial RT-PCR results and serial CT imaging, and serial CT changes in patients with COVID-19 pneumonia.
Materials and methods
Patient population and clinical arrangement
The institutional review board of our hospital approved this retrospective study (FK20202815), and informed consent was waived. From January 21 to February 18, 2020, patients who were suspected of having novel coronavirus infection and confirmed were retrospectively enrolled in our study. All patients were confirmed by laboratory virus nucleic acid test (real-time fluorescence polymerase chain reaction revealed positive detection of COVID-19, RT-PCR assays using throat swab samples) according to the guideline of the Diagnosis and Treatment of Pneumonia Caused by COVID-19 (Trial Version 3) published by the Chinese government [5]. COVID-19 infection was diagnosed as soon as any one of the nucleic acid test results was positive. Chest CT imaging was also conducted to determine whether pneumonia was present. All confirmed patients underwent serial RT-PCR assays and serial chest CT imaging to provide evidence to treat the disease. The RT-PCR test was repeated every 3 to 5 days to monitor the dynamic conversion of the RT-PCR results (negative to positive, positive to negative). The RT-PCR results were reviewed in our hospital information system (HIS). We compared the differences in the time points of both methods suggesting the diagnosis of COVID-19 infection.
The RT-PCR assays were conducted with TaqMan One-Step RT-PCR kits obtained from Huirui Biotechnology Co., Ltd., which were approved by the China Food and Drug Administration (CFDA).
The chest CT protocol
Chest CTs were conducted every 2 to 7 days to confirm the presence of pneumonia and monitor the changes in lung appearance according to the guideline of the Diagnosis and Treatment of Pneumonia Caused by COVID-19 (Trial Version 3) published by the Chinese government [5]. All images were acquired on 2 CT scanners (Brilliance iCT, Philips Healthcare, and Aqulion 64, Toshiba) using the following parameters: tube voltage, 120 kVp; automatic tube current modulation; pitch, 0.76–1.22; matrix = 512 × 512; field of volume = 400 × 400 mm; and slice thickness, 5.0 mm. All images were reconstructed with slice thickness of 1.0 mm at the same increment. Lung and mediastinum windows were simultaneously reconstructed.
Image interpretation and analysis
Chest imaging was analyzed by two radiologists, blinded to RT-PCR results. The epidemiological history and clinical symptoms were obtained when they reviewed the images. The time point of the first positive lung CT (after the onset of the initial symptoms) and serial CT features and changes were subsequently recorded.
The CT features of new coronavirus-infected pneumonitis included GGO, consolidation, crazy paving patterns (inter- or intra-lobular septal thickening superimposed on GGO), fibrotic lesions, air bronchogram signs, small-vessel thickening, and pleural effusion according to expert recommendations from the Chinese Society of Radiology [12]. We divided CT findings into main features (GGO, consolidation, crazy paving patterns, and fibrotic lesions) and secondary features (air bronchogram signs, small-vessel thickening, and pleural effusion). The number of lung lobes involved and distribution of lung abnormalities were also evaluated. The lesion distribution was classified into sub-pleural areas (predominantly the peripheral one third of the lung involved), central areas, and sub-pleural plus central areas.
CT imaging progression presented as increasing number, size, extent and density of GGO, consolidation and crazy paving patterns, or GGO evolving to consolidation and crazy paving patterns [7, 11, 13]. The smaller size, number, extent, and density of these signs predicted COVID-19 pneumonia improvement [13,14,15,16]. The relationship between the RT-PCR results with chest imaging changes was analyzed.
Therapeutic approaches
General treatment included supportive care, monitoring routine blood, urinalysis, biochemistry, coagulation function, chest CT, oxygen therapy, and antiviral therapy. Respiratory and circulation support were used for severe patients.
Discharge criteria
The nucleic acid test and CT imaging were following up to the time of discharge. According to the guideline of the Diagnosis and Treatment of Pneumonia Caused by COVID-19 (Trial Version 3) published by the Chinese government [5], the criteria of hospital discharge met all of the following conditions: (1) body temperature returned to normal for at least 3 days; (2) respiratory symptoms markedly improved; (3) chest imaging distinctly improved; and (4) continuous RT-PCR assays with sputum samples or nasopharyngeal or throat swab samples converted to negative at 2 time intervals of at least 24 h.
Statistical analysis
Continuous variables were presented as mean ± standard deviation, and categorical variables were expressed as frequencies and percentages. The t test was used to determine the differences in the time of RT-PCR first positive results and chest CT first positive findings (after the onset of the initial symptoms), the time of RT-PCR conversion to negative and CT finding improvements (after the onset of the initial symptoms). The chi-squared test and Fisher’s exact test were performed to compare the changes in CT features, number of lung lobes involved, and lesion distribution on serial CT images. A two-tailed p value < 0.05 was considered statistically significant. All statistical analyses were conducted using SPSS version 21.0 (SPSS Inc).
Results
Study population
From January 21 to February 18, 2020, 155 consecutive patients confirmed with COVID-19 pneumonia (87 males and 68 females aged from 9 months to 82 years, mean age 42.97 ± 14.85 years, including 4 pediatric patients aged 9 months, 6 years, 6.5 years, and 8 years respectively) were included in this study. All patients had history of Wuhan exposure or close contact with the patients confirmed or highly suspected of COVID-19 infection within 2 weeks before the onset of illness. The initial symptoms included fever, cough, chest distress, shortness of breath, fatigue, and diarrhea. The initial laboratory examinations showed decreased lymphocyte percentages and increased C-reactive protein and erythrocyte sedimentation rates. The clinical data are summarized in Table 1. All patients were discharged upon meeting the discharge criteria and continuously quarantined at home after an average hospitalization period of 17.27 ± 5.68 days (range, 10–30 days).
The time comparison between serial RT-PCR results and chest CT findings
All patients underwent RT-PCR assays 3 to 7 times with time intervals of 3 to 5 days (average 4.5 days), and chest CT scans 4 to 6 times (4 times for every pediatric patient) with time intervals of 2 to 7 days (average 4.7 days). Numbers of patients with CT abnormality and who were RT-PCR positive at each day are listed in Table 2.
The initial RT-PCR positive results and lung CT abnormality time, RT-PCR conversion to negative time, and CT finding improvement time (after the onset of initial symptoms) are summarized in Table 3. A comparison of the time points of the first RT-PCR positive results and the positive imaging time demonstrated significant differences between the two methods for detecting COVID-19 infection (t = − 7.31, p = 0.000) with CTs 2.61 days earlier than the nucleic acid test. The lung CT improvement time was significantly shorter than the RT-PCR conversion to negative time (t = − 4.72, p = 0.000).
Serial chest CT image evaluation
Based on the degree of lung involvement and CT findings, three stages were identified from the onset of initial symptoms: stage 1 (0–3 days), stage 2 (4–7 days), and stage 3 (8–14 days and later).
The typical course of COVID-19 pneumonia is demonstrated by serial chest CT images (Figs. 1, 2, and 3). The CT characteristics are summarized in Table 4. In the main CT features, GGO was predominant in stage 1, then consolidation and crazy paving signs dramatically increased in stage 2. In stage 3, fibrotic lesion growth markedly increased, accompanied by consolidation and crazy paving signs (Fig. 4). There were significant differences in the main CT features among the different stages (χ2 = 219.00, p = 0.000). The comparison among groups also demonstrated significant differences between stages 1 and 2 (χ2 = 155.98, p = 0.000), stages 1 and 3 (χ2 = 186.45, p = 0.000), and stages 2 and 3 (χ2 = 16.99, p = 0.001).
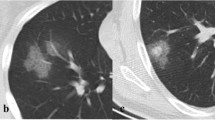
A 42-year-old female patient presented with fever for 2 days. a The baseline CT scan (2 days after initial symptom, stage 1) obtained 2 days before the first positive RT-PCR test showed discrete GGO and consolidation in her bilateral lower lobes. b The follow-up CT 2 days later (day 4, stage 2) showed enlarged patchy consolidation in her bilateral lower lobes, accompanied by air bronchogram signs. New GGO was detected in the right middle lobe and left lingual segment. c The follow-up CT 5 days later (day 9, stage 3) obtained 2 days before RT-PCR converting to negative showed that the size of consolidation decreased. d Another 3 days later (day 12, stage 3), CT scan demonstrated that the lesions were further reduced in size and fibrotic lesions were predominant
A 56-year-old male patient presented with fever and cough for 2 days. a The baseline CT scan (day 2, stage 1) obtained 5 days before the first positive RT-PCR test showed discrete GGO in his bilateral upper lobes and right lower lobe. Small-vessel thickening was detected in the lesions in the left upper lobe. b The follow-up CT 3 days later (day 5, stage 2) showed that the size of GGO was slightly reduced in the bilateral upper lobes. c The follow-up CT 4 days later (day 9, stage 3) obtained 2 days before RT-PCR converting to negative showed that the size of lesions further decreased. d Another 4 days later (day 13, stage 3), a CT scan demonstrated that the lesions were further improved and fibrotic lesions were predominant
A 26-year-old female patient presented with fever for 1 day. a The baseline CT scan (day 1, stage 1) obtained 3 days before the first positive RT-PCR test showed discrete GGO in her bilateral lower lobes. b The follow-up CT 2 days later (day 3, stage 1) showed that the size of GGO was markedly enlarged in the left lower lobe, accompanied by small-vessel thickening. c The follow-up CT obtained 3 days later (day 6, stage 2) showed that the lesions had progressed to consolidation in the left lower lobes and new GGO was identified in the right lower lobe. Crazy paving signs were detected in the left lower lobe. d Another 3 days later (day 9, stage 3), a CT scan showed lesion progression in the right lower lobe, accompanied by crazy paving signs. e The follow-up CT scan 3 days later (day 12, stage 3), 3 days before RT-PCR converting to negative, showed that the size of the lesions decreased, fibrotic lesions were predominant in the right lower lobe, and crazy paving signs dominated the left lower lesion. f Another 3 days later (day 15, stage 3), a CT scan demonstrated that the lesions were further improved and fibrotic lesions left
Among the secondary CT features, small-vessel thickening dominated stage 1. Air bronchogram signs gradually increased in stages 2 and 3. Pleural effusion was rare throughout the disease course (Fig. 5). There were significant differences in the secondary CT features among the different stages (χ2 = 48.58, p = 0.000). The comparison among groups also demonstrated significant differences between stages 1 and 2 (χ2 = 23.87, p = 0.000) and stages 1 and 3 (χ2 = 48.10, p = 0.000), but no differences between stages 2 and 3 (χ2 = 3.70, p = 0.157).
The changes in the number of lung lobes involved on serial CT scans are demonstrated in Fig. 6. For the number of lung lobes involved, there were statistically significant differences among the different stages (χ2 = 29.30, p = 0.001). The comparison among groups demonstrated statistically significant differences between stages 1 and 3 (χ2 = 25.55, p = 0.000), but no differences between stages 1 and 2 (χ2 = 9.29, p = 0.098) and stages 2 and 3 (χ2 = 8.55, p = 0.129).
There were statistically significant differences in lesion distribution among the different stages (χ2 = 50.99, p = 0.000). The comparison among groups demonstrated significant differences between stages 1 and 2 (p = 0.000) and stages 1 and 3 (p = 0.000), but no differences between stages 2 and 3 (χ2 = 0.841, p = 0.359).
Discussion
In this study, we evaluated the temporal relationship between the appearance and resolution of CT findings and the RT-PCR evidence of COVID-19 virus presence and investigated lung imaging changes in COVID-19 pneumonia over time. This study’s major findings were twofold: first, the initial chest CT abnormality time was significantly shorter than the initial RT-PCR positive results, and the lung CT improvement time was shorter than the RT-PCR conversion to negative; second, chest CT is a reliable, non-invasive, rapid tool to monitor the occurrence, deterioration, and improvements in COVID-19 pneumonia.
The confirmation of COVID-19 infection depended on viral nucleic acid test using RT-PCR assays, which determined whether patients should be hospitalized or quarantine at home. But the RT-PCR test had distinct limitations due to its low sensitivity, insufficient stability, and relatively long processing time to obtain results. Some studies reported that the positive rate of RT-PCR assays for throat swab samples was 30–60% [17, 18]. Many factors can affect the RT-PCR results, including sampling operations, specimen sources (upper or lower respiratory tract), sampling timing, and the detection kit’s performance [18]. A recent study showed that the salivary viral load was highest during the first week after symptom onset [19], which could account for the rapid spread of this pandemic at the early stage. Patients without timely isolation and therapy due to initial negative RT-PCR results definitely cause COVID-19 to spread.
Chest CT is a non-invasive, rapid, convenient imaging diagnostic tool and can detect mild lung abnormalities at the early stage of COVID-19 pneumonia. Our study showed that the initial chest CT abnormality time (3.23 ± 3.04 days) was significantly shorter than that of the initial RT-PCR positive results (5.84 ± 3.23 days). Our study was in agreement with previous research [6] that revealed that 29% (15/51) of patients had negative RT-PCR and positive CT results at initial presentation and 2% (1/51) demonstrated the opposite [6]. Therefore, CT plays a vital role in the early detection of COVID-19 pneumonia, especially for patients with initial negative RT-PCR results. However, the specificity of CT for diagnosing COVID-19 pneumonia varies. Tao et al [17] reported that the sensitivity and specificity of chest CT for indicating COVID-19 infection was 97% and 25%, respectively. Bai et al reported that seven radiologists demonstrated sensitivities of 80%, 67%, 97%, 93%, 83%, 73%, and 70% and specificities of 100%, 93%, 7%, 100%, 93%, 93%, and 100% [20]. Although the RT-PCR test is the gold standard for the diagnosis of COVID-19 infection, chest CT examination is essential for the early identification of potential patients and helps to determine treatment strategies. COVID-19 infection was upgraded from epidemic to pandemic by the World Health Organization (WHO) on March 11, 2020 [21]. Thus, chest CT will certainly play a crucial role in the early detection of the disease to contain the pandemic’s spread.
Our study also found that the lung CT improvement time (11.58 ± 4.59 days) was significantly shorter than that of RT-PCR conversion to negative (14.38 ± 5.78 days). This indicates that CT is very useful for monitoring the course of COVID-19 pneumonia to direct clinicians to adjust the therapeutic strategy.
In our study, dynamic serial chest CT examination (4–6 times) with a relatively large patient population provided reliable data to observe the disease course. We divided the disease into 3 stages according to the time since the onset of the initial symptoms. At the early stage, the CT features of COVID-19 are predominantly GGO and small-vessel thickening. GGO is the main CT feature, and small-vessel thickening is the secondary feature. As the disease progresses, GGO evolves to consolidation. Crazy paving signs are also markedly enhanced as the disease progresses. At stage 2, air bronchogram signs are also dramatically increased as secondary signs of consolidation. Small-vessel thickening is still abundant as the secondary sign of GGO and consolidation. We also noticed that fibrotic lesions were gradually added, which represents the reparative process. At stage 3, fibrotic lesions significantly increased, accompanied by more consolidation, GGO, and crazy paving signs. This indicates the reparative and progressive process of COVID-19 pneumonia simultaneously, and various CT features coexist at stage 3. Our study demonstrated that the lung improvement time was 11.58 ± 4.59 days. The CT feature changes during follow-up in our study were in agreement with other reports [22, 23]. The recently published proposed COVID-RADS and common lexicon would improve the communication of imaging findings and facilitate the diagnosis and management of COVID-19 patients [23].
The lung CT appearance may be consistent with the pathology. The autopsy and histological examination of COVID-19 pneumonia showed bilateral diffuse alveolar damage with proteinaceous exudate, cellular fibromyxoid exudate, pulmonary edema, reactive hyperplasia of pneumocytes, desquamation of pneumocytes, hyaline membrane formation, fibroblastic plugs in airspaces, and interstitial mononuclear inflammatory infiltration [24, 25], which may be in accordance with the appearance of GGO, consolidation, and crazy paving signs. Tian et al reported patchy pneumocyte hyperplasia and interstitial thickening indicating an ongoing reparative process at the early stage of COVID-19 pneumonia [25]. Notably, when the patients met the discharge criteria, there was still patchy consolidation left in the lung. This suggests that patients should quarantine at home for an additional period (2 weeks in China) after hospital discharge.
Our study also showed that typical COVID-19 pneumonia started as unilateral or bilateral sub-pleural GGO, then evolved to consolidation involving the sub-pleural and central areas. We also found that 12.9% of patients demonstrated normal chest CT images at the early stage. Then the number of lung lobes involved increased at stage 2. However, the number of lung lobes involved remained high at stage 3 and demonstrated no significant differences compared to stage 2. Residual GGO, scattered consolidation, and sub-pleural parenchymal bands persisted at stage 3. Thus, patients still need to be followed up after hospital discharge.
This study had limitations that merit mention. First, this is a short-term retrospective study, and long-term radiological follow-up is needed to monitor the pulmonary outcome due to the novel coronavirus infection. Second, this study lacked severe COVID-19 pneumonia patients (including respiratory failure requiring mechanical ventilation, shock, multiple organ failure necessitating ICU care) [5], so the changes in these patients’ CT findings need to be investigated in future research. Third, the CT scans’ radiation doses were not evaluated, but it is more important to detect infected patients, understand the disease course, manage patients, and contain the pandemic.
In conclusion, chest CT plays a vital role in the early detection of COVID-19 pneumonia, monitoring lesion progression, and improving treatment strategies. Our results indicated that CT showed lung abnormalities earlier than the time of initial positive nucleic acid tests with RT-PCR for the detection of COVID-19 infection, and demonstrated lung improvements earlier than the RT-PCR conversion to negative time. Chest CT should be considered as a useful tool to perform in the early stage of suspected COVID-19 infection, especially during the pandemic.
Abbreviations
- CFDA:
-
China Food and Drug Administration
- COVID-19:
-
Coronavirus disease 2019
- CT:
-
Computed tomography
- GGO:
-
Ground glass opacity
- HIS:
-
Hospital information system
- NHCPRC:
-
National Health Commission of the People’s Republic of China
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
References
Huang C, Wang Y, Li X et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223):497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
Phan LT, Nguyen TV, Luong QC et al (2020) Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med 382(9):872–874. https://doi.org/10.1056/NEJMc2001272
Chan JF, Yuan S, Kok KH et al (2020) A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 395(10223):514–523. https://doi.org/10.1016/S0140-6736(20)30154-9
World Health Organization (2020) WHO Director-General’s remark at the media briefing on 2019-nCoV on 27 March 2020. at https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncon-on-9-march-2020. Published March 09, 2020
National Health Commission of the People’s Republic of China. Diagnosis and treatment protocols of pneumonia caused by a novel coronavirus (trial version 3). http://www.nhc.gov.cn/yzygj/s7653p202001/f492c9153ea9437bb587ce2ffcbee1fa/files/39e7578d85964dbe81117736dd789d8f.pdf Published on January 22, 2020
Fang Y, Zhang H, Xie J et al (2020) Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology 296(2):E115–E117. https://doi.org/10.1148/radiol.2020200432
Chung M, Bernheim A, Mei X et al (2020) CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology. 295(1):202–207. https://doi.org/10.1148/radiol.2020200230
Zu ZY, Jiang MD, Xu PP et al (2020) Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology. 296(2):E15–E25. https://doi.org/10.1148/radiol.2020200490
Fang Y, Zhang H, Xu Y, Xie J, Pang P, Ji W (2020) CT manifestations of two cases of 2019 novel coronavirus (2019-nCov). Radiology 295(1):208–209. https://doi.org/10.1148/radiol.2020200280
Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J (2020) Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology 296(2):E41–E45. https://doi.org/10.1148/radiol.2020200343
Pan F, Ye T, Sun P et al (2020) Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology 295(3):715–721. https://doi.org/10.1148/radiol.2020200370
Chinese Society of Radiology (2020) Radiological diagnosis of new coronavirus infected pneumonitis: expert recommendation from the Chinese Society of Radiology (first edition). Chin J Radiol 54(00):E001–E001. https://doi.org/10.3760/cma.j.issn.1005-1201.2020.0001
Song F, Shi N, Shan F et al (2020) Emerging coronavirus 2019-nCoV pneumonia. Radiology 295(1):210–217. https://doi.org/10.1148/radiol.2020200274
Wang D, Hu B, Hu C et al (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 323(11):1061–1069. https://doi.org/10.1001/jama.2020.1585
Shi H, Han X, Zheng C (2020) Evolution of CT manifestations in a patient recovered novel coronavirus (2019-nCoV) pneumonia in Wuhan, China. Radiology 295(1):20. https://doi.org/10.1148/radiol.2020200269
Duan YN, Qin J (2020) Pre- and posttreatment chest CT findings: 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology 295(1):21. https://doi.org/10.1148/radiol.2020200323
Ai T, Yang Z, Hou H et al (2020) Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology 296(2):E32–E40. https://doi.org/10.1148/radiol.2020200642
Yang Y, Yang M, Shen C et al (2020) Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. medRxiv preprint. https://doi.org/10.1101/2020.02.11.20021493
To KKW, Tsang OTY, Leung WS et al (2020) Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 20(5):565–574. https://doi.org/10.1016/S1473-3099(20)30196-1
Bai HX, Hsieh B, Xiong Z et al (2020) Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology 296(2):E46–E54. https://doi.org/10.1148/radiol.2020200823
World Health Organization (2020) WHO Director-General’s opening remarks at the media briefing on 2019-nCoV on 9 March 2020. at https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncon-on-9-march-2020. Published March 09, 2020
Hani C, Trieu NH, Saab I et al (2020) COVID-19 pneumonia: a review of typical CT findings and differential diagnosis. Diagn Interv Imaging 101(5):263–268. https://doi.org/10.1016/j.diii.2020.03.014
Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A (2020) Coronavirus disease 2019 (COVID-19) imaging reporting and data system (COVID-RADS) and common lexicon: a proposal based on the imaging data of 37 studies. Eur Radiol 28:1–13. https://doi.org/10.1007/s00330-020-06863-0
Xu Z, Shi L, Wang YJ et al (2020) Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 8(4):420–422. https://doi.org/10.1016/S2213-2600(20)30076-X
Tian SF, Hu WD, Niu L, Liu H, Xu H, Xiao S (2020) Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol 15(5):700–704. https://doi.org/10.1016/j.jtho.2020.02.010
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Tao Li.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gu, J., Yang, L., Li, T. et al. Temporal relationship between serial RT-PCR results and serial chest CT imaging, and serial CT changes in coronavirus 2019 (COVID-19) pneumonia: a descriptive study of 155 cases in China. Eur Radiol 31, 1175–1184 (2021). https://doi.org/10.1007/s00330-020-07268-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07268-9