Abstract
Objectives
To identify multiparametric MRI biomarkers to predict the tumor response to neoadjuvant FOLFIRINOX therapy in patients with borderline resectable (BR) or locally advanced (LA) pancreatic ductal adenocarcinoma (PDAC).
Methods
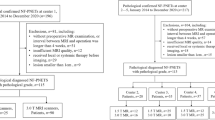
From May 2016 to March 2018, adult patients with BR or LA PDAC were prospectively enrolled in this study. They received eight cycles of FOLFIRINOX therapy and underwent multiparametric MRI twice (at baseline and after the second cycle). MRI evaluations included dynamic contrast-enhanced MRI, intravoxel incoherent motion diffusion-weighted imaging, and assessment of T2* relaxivity (R2*) and the change in T1 relaxivity (ΔR1, equilibrium phase R1 minus non-enhanced R1) of the tumors. Factors to predict the responders determined by the best overall response during FOLFIRINOX therapy and those to predict progression-free survival (PFS) and overall survival (OS) were evaluated using multivariable logistic regression and the Cox proportional hazard model.
Results
Forty-one patients (mean age, 60.3 years ± 9.3; 24 men) were included. Among the clinical and MRI factors, the baseline ΔR1 (adjusted odds ratio, 31.07; p = 0.008) was the only independent predictor for tumor response. The baseline ΔR1 was also an independent predictor for PFS (adjusted hazard ratio, 0.40; p = 0.033) along with R0 resection. The use of a cutoff ΔR1 value of ≥ 1.31 s-1 enabled prognostic stratification (median PFS, 16.0 months vs.10.0 months; p = 0.029; median OS, 34.9 months vs. 16.6 months; p = 0 .023, respectively).
Conclusions
The baseline tumor ΔR1 value may be useful to predict tumor response and survival in patients with BR or LA PDAC receiving FOLFIRINOX neoadjuvant therapy.
Key Points
• Baseline ΔR1 was an independent predictor for tumor response (adjusted odds ratio, 31.07; p = 0.008) and progression-free survival (adjusted hazard ratio, 0.40; p = 0.033) in patients with borderline resectable or locally advanced pancreatic ductal adenocarcinoma receiving neoadjuvant FOLFIRINOX therapy.
• The criterion of baseline ΔR1 value ≥ 1.31 s -1 allowed for the prediction of favorable tumor response and survival outcome after neoadjuvant FOLFIRINOX therapy.




Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUC:
-
Area under the receiver operating characteristic curve
- BR:
-
Borderline resectable
- CAIPIRINHA-VIBE:
-
Volume-interpolated breath-hold examination with controlled aliasing in parallel imaging results in higher acceleration
- CI:
-
Confidence interval
- CR:
-
Complete response
- DCE:
-
Dynamic contrast-enhanced
- D fast :
-
Fast diffusion coefficient
- D slow :
-
Slow diffusion coefficient
- f :
-
Perfusion fraction
- HR:
-
Hazard ratio
- iAUC:
-
Initial area under the curve in 60 s
- ICC:
-
Intraclass correlation coefficient
- IVIM-DWI:
-
Intravoxel incoherent motion diffusion-weighted imaging
- k ep :
-
Rate constant between extracellular extravascular space and plasma
- K trans :
-
Volume transfer coefficient
- LA:
-
Locally advanced
- OR:
-
Odds ratio
- OS:
-
Overall survival
- PD:
-
Progressive disease
- PDAC:
-
Pancreatic ductal adenocarcinoma
- PFS:
-
Progression-free survival
- PR:
-
Partial response
- R1:
-
T1 relaxivity
- R2*:
-
T2* relaxivity
- RECIST:
-
Response evaluation criteria in solid tumors
- ROI:
-
Region-of-interest
- SD:
-
Stable disease
- TE:
-
Echo time
- v e :
-
Fractional extracellular extravascular space volume
- ΔR1:
-
Change in T1 relaxivity
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Rawla P, Sunkara T, Gaduputi V (2019) Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol 10:10–27
Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS (2007) National failure to operate on early stage pancreatic cancer. Ann Surg 246:173–180
Ferrone CR, Marchegiani G, Hong TS et al (2015) Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg 261:12–17
Heestand GM, Murphy JD, Lowy AM (2015) Approach to patients with pancreatic cancer without detectable metastases. J Clin Oncol 33:1770–1778
Tempero MA, Malafa MP, Chiorean EG et al (2019) Pancreatic Adenocarcinoma, Version 1.2019. J Natl Compr Canc Netw 17:202–210
Conroy T, Desseigne F, Ychou M et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825
Fukukura Y, Takumi K, Higashi M et al (2014) Contrast-enhanced CT and diffusion-weighted MR imaging: performance as a prognostic factor in patients with pancreatic ductal adenocarcinoma. Eur J Radiol 83:612–619
Hamdy A, Ichikawa Y, Toyomasu Y et al (2019) Perfusion CT to assess response to neoadjuvant chemotherapy and radiation therapy in pancreatic ductal adenocarcinoma: initial experience. Radiology 292:628–635
Kim JH, Park SH, Yu ES et al (2010) Visually isoattenuating pancreatic adenocarcinoma at dynamic-enhanced CT: frequency, clinical and pathologic characteristics, and diagnosis at imaging examinations. Radiology 257:87–96
Lee S, Kim SH, Park HK, Jang KT, Hwang JA, Kim S (2018) Pancreatic ductal adenocarcinoma: rim enhancement at mr imaging predicts prognosis after curative resection. Radiology 288:456–466
Park MS, Klotz E, Kim MJ et al (2009) Perfusion CT: noninvasive surrogate marker for stratification of pancreatic cancer response to concurrent chemo- and radiation therapy. Radiology 250:110–117
Lee HJ, Im DJ, Youn JC et al (2016) Myocardial extracellular volume fraction with dual-energy equilibrium contrast-enhanced cardiac CT in nonischemic cardiomyopathy: a prospective comparison with cardiac MR imaging. Radiology 280:49–57
Koay EJ, Truty MJ, Cristini V et al (2014) Transport properties of pancreatic cancer describe gemcitabine delivery and response. J Clin Invest 124:1525–1536
Bandula S, Punwani S, Rosenberg WM et al (2015) Equilibrium contrast-enhanced CT imaging to evaluate hepatic fibrosis: initial validation by comparison with histopathologic sampling. Radiology 275:136–143
O'Connor JPB, Robinson SP, Waterton JC (2019) Imaging tumour hypoxia with oxygen-enhanced MRI and BOLD MRI. Br J Radiol 92:20180642
Jing X, Yang F, Shao C et al (2019) Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer 18:157
Yoo C, Lee SS, Song KB et al (2020) Neoadjuvant modified FOLFIRINOX followed by postoperative gemcitabine in borderline resectable pancreatic adenocarcinoma: a Phase 2 study for clinical and biomarker analysis. Br J Cancer 123:362–368
Wang HZ, Riederer SJ, Lee JN (1987) Optimizing the precision in T1 relaxation estimation using limited flip angles. Magn Reson Med 5:399–416
Tofts PS, Brix G, Buckley DL et al (1999) Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 10:223–232
Tofts PS, Kermode AG (1991) Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson Med 17:357–367
Sung YS, Park B, Choi Y et al (2016) Dynamic contrast-enhanced MRI for oncology drug development. J Magn Reson Imaging 44:251–264
Orton MR, d'Arcy JA, Walker-Samuel S et al (2008) Computationally efficient vascular input function models for quantitative kinetic modelling using DCE-MRI. Phys Med Biol 53:1225–1239
Kim SY, Lee SS, Byun JH et al (2010) Malignant hepatic tumors: short-term reproducibility of apparent diffusion coefficients with breath-hold and respiratory-triggered diffusion-weighted MR imaging. Radiology 255:815–823
Lee JH, Cheong H, Lee SS et al (2016) Perfusion assessment using intravoxel incoherent motion-based analysis of diffusion-weighted magnetic resonance imaging: validation through phantom experiments. Invest Radiol 51:520–528
Tempero MA, Malafa MP, Al-Hawary M et al (2017) Pancreatic adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 15:1028–1061
Baliyan V, Kordbacheh H, Parakh A, Kambadakone A (2018) Response assessment in pancreatic ductal adenocarcinoma: role of imaging. Abdom Radiol (NY) 43:435–444
Zhao Q, Rashid A, Gong Y et al (2012) Pathologic complete response to neoadjuvant therapy in patients with pancreatic ductal adenocarcinoma is associated with a better prognosis. Ann Diagn Pathol 16:29–37
Cassinotto C, Mouries A, Lafourcade JP et al (2014) Locally advanced pancreatic adenocarcinoma: reassessment of response with CT after neoadjuvant chemotherapy and radiation therapy. Radiology 273:108–116
Katz MH, Fleming JB, Bhosale P et al (2012) Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer 118:5749–5756
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Wagner M, Antunes C, Pietrasz D et al (2017) CT evaluation after neoadjuvant FOLFIRINOX chemotherapy for borderline and locally advanced pancreatic adenocarcinoma. Eur Radiol 27:3104–3116
Bewick V, Cheek L, Ball J (2004) Statistics review 13: receiver operating characteristic curves. Crit Care 8:508–512
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–163
Fukukura Y, Kumagae Y, Higashi R et al (2020) Extracellular volume fraction determined by equilibrium contrast-enhanced dual-energy CT as a prognostic factor in patients with stage IV pancreatic ductal adenocarcinoma. Eur Radiol 30:1679–1689
Akisik MF, Sandrasegaran K, Bu G, Lin C, Hutchins GD, Chiorean EG (2010) Pancreatic cancer: utility of dynamic contrast-enhanced MR imaging in assessment of antiangiogenic therapy. Radiology 256:441–449
Cuneo KC, Chenevert TL, Ben-Josef E et al (2014) A pilot study of diffusion-weighted MRI in patients undergoing neoadjuvant chemoradiation for pancreatic cancer. Transl Oncol 7:644–649
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Korea government (MSIT) (No. 2020R1F1A1048826).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Seung Soo Lee.
Conflict of Interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Prospective
• Diagnostic or prognostic study
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 47 kb)
Rights and permissions
About this article
Cite this article
Kang, J.H., Lee, S.S., Kim, J.H. et al. Multiparametric MRI for prediction of treatment response to neoadjuvant FOLFIRINOX therapy in borderline resectable or locally advanced pancreatic cancer. Eur Radiol 31, 864–874 (2021). https://doi.org/10.1007/s00330-020-07134-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07134-8