Abstract
Objectives
To investigate the predictive value of quantifiable imaging and inflammatory biomarkers in patients with hepatocellular carcinoma (HCC) for the clinical outcome after drug-eluting bead transarterial chemoembolization (DEB-TACE) measured as volumetric tumor response and progression-free survival (PFS).
Methods
This retrospective study included 46 patients with treatment-naïve HCC who received DEB-TACE. Laboratory work-up prior to treatment included complete and differential blood count, liver function, and alpha-fetoprotein levels. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were correlated with radiomic features extracted from pretreatment contrast-enhanced magnetic resonance imaging (MRI) and with tumor response according to quantitative European Association for the Study of the Liver (qEASL) criteria and progression-free survival (PFS) after DEB-TACE. Radiomic features included single nodular tumor growth measured as sphericity, dynamic contrast uptake behavior, arterial hyperenhancement, and homogeneity of contrast uptake. Statistics included univariate and multivariate linear regression, Cox regression, and Kaplan–Meier analysis.
Results
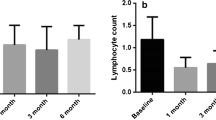
Accounting for laboratory and clinical parameters, high baseline NLR and PLR were predictive of poorer tumor response (p = 0.014 and p = 0.004) and shorter PFS (p = 0.002 and p < 0.001). When compared to baseline imaging, high NLR and PLR correlated with non-spherical tumor growth (p = 0.001 and p < 0.001).
Conclusions
This study establishes the prognostic value of quantitative inflammatory biomarkers associated with aggressive non-spherical tumor growth and predictive of poorer tumor response and shorter PFS after DEB-TACE.
Key Points
• In treatment-naïve hepatocellular carcinoma (HCC), high baseline platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR) are associated with non-nodular tumor growth measured as low tumor sphericity.
• High PLR and NLR are predictive of poorer volumetric enhancement-based tumor response and PFS after DEB-TACE in HCC.
• This set of readily available, quantitative immunologic biomarkers can easily be implemented in clinical guidelines providing a paradigm to guide and monitor the personalized application of loco-regional therapies in HCC.





Similar content being viewed by others
Abbreviations
- AFP:
-
Alpha-fetoprotein
- ALC:
-
Absolute lymphocyte count
- ALT:
-
Alanine aminotransferase
- ANC:
-
Absolute neutrophil count
- AP:
-
Alkaline phosphatase
- AST:
-
Aspartate aminotransferase
- BCLC:
-
Barcelona Clinic Liver Cancer
- DEB:
-
Drug-eluting bead
- ETB:
-
Enhancing tumor burden
- HCC:
-
Hepatocellular carcinoma
- HIPAA:
-
Health Insurance Portability and Accountability Act
- LI-RADS:
-
Liver Imaging Reporting and Data System
- NLR:
-
Neutrophil-to-lymphocyte ratio
- PFS:
-
Progression-free survival
- PLR:
-
Platelet-to-lymphocyte ratio
- qEASL:
-
Quantitative European Association for the Study of the Liver
- TACE:
-
Transarterial chemoembolization
- TB:
-
Tumor burden
- TME:
-
Tumor microenvironment
- TTV:
-
Total tumor volume
- VEGF:
-
Vascular endothelial growth factor
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Crissien AM, Frenette C (2014) Current management of hepatocellular carcinoma. Gastroenterol Hepatol (N Y) 10:153–161
Song JE, Kim DY (2017) Conventional vs drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma. World J Hepatol 9:808–814
Baur J, Ritter CO, Germer CT, Klein I, Kickuth R, Steger U (2016) Transarterial chemoembolization with drug-eluting beads versus conventional transarterial chemoembolization in locally advanced hepatocellular carcinoma. Hepat Med 8:69–74
Dufour JF, Bargellini I, De Maria N, De Simone P, Goulis I, Marinho RT (2013) Intermediate hepatocellular carcinoma: current treatments and future perspectives. Ann Oncol 24(Suppl 2):ii24–ii29
European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer (2012) EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 56:908–943
Bruix J, Han KH, Gores G, Llovet JM, Mazzaferro V (2015) Liver cancer: approaching a personalized care. J Hepatol 62:S144–S156
Forner A, Reig M, Bruix J (2018) Hepatocellular carcinoma. Lancet 391:1301–1314
Bolondi L, Burroughs A, Dufour JF et al (2012) Heterogeneity of patients with intermediate (BCLC B) hepatocellular carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis 32:348–359
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Lambin P, Leijenaar RTH, Deist TM et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14:749–762
Roth GS, Decaens T (2017) Liver immunotolerance and hepatocellular carcinoma: patho-physiological mechanisms and therapeutic perspectives. Eur J Cancer 87:101–112
Prieto J, Melero I, Sangro B (2015) Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 12:681–700
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420:860–867
Unitt E, Rushbrook SM, Marshall A et al (2005) Compromised lymphocytes infiltrate hepatocellular carcinoma: the role of T-regulatory cells. Hepatology 41:722–730
Kuang DM, Zhao Q, Wu Y et al (2011) Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol 54:948–955
Bambace NM, Holmes CE (2011) The platelet contribution to cancer progression. J Thromb Haemost 9:237–249
Zhou DS, Xu L, Luo YL et al (2015) Inflammation scores predict survival for hepatitis B virus-related hepatocellular carcinoma patients after transarterial chemoembolization. World J Gastroenterol 21:5582–5590
Zheng J, Cai J, Li H et al (2017) Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as prognostic predictors for hepatocellular carcinoma patients with various treatments: a meta-analysis and systematic review. Cell Physiol Biochem 44:967–981
American College of Radiology (2018) American College of Radiology: liver imaging reporting and data system. American College of Radiology, Virginia Available via https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CT-MRI-LI-RADS-v2018. Accessed 7 Feb 2020
Tacher V, Lin M, Duran R et al (2016) Comparison of existing response criteria in patients with hepatocellular carcinoma treated with transarterial chemoembolization using a 3D quantitative approach. Radiology 278:275–284
van Griethuysen JJM, Fedorov A, Parmar C et al (2017) Computational radiomics system to decode the radiographic phenotype. Cancer Res 77:e104–e107
Heinze G, Wallisch C, Dunkler D (2018) Variable selection - a review and recommendations for the practicing statistician. Biom J 60:431–449
Kim HC, Suk KT, Kim DJ et al (2014) Transarterial chemoembolization in Barcelona Clinic Liver Cancer stage 0/A hepatocellular carcinoma. World J Gastroenterol 20:745–754
Nelen SD, Verhoeven RHA, Lemmens VEPP, de Wilt JHW, Bosscha K (2017) Increasing survival gap between young and elderly gastric cancer patients. Gastric Cancer 20:919–928
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Buonaguro L, Tagliamonte M, Petrizzo A, Damiano E, Tornesello ML, Buonaguro FM (2015) Cellular prognostic markers in hepatocellular carcinoma. Future Oncol 11:1591–1598
O'Rourke JM, Sagar VM, Shah T, Shetty S (2018) Carcinogenesis on the background of liver fibrosis: implications for the management of hepatocellular cancer. World J Gastroenterol 24:4436–4447
Gajewski TF, Schreiber H, Fu YX (2013) Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 14:1014–1022
Rai V, Abdo J, Alsuwaidan AN, Agrawal S, Sharma P, Agrawal DK (2018) Cellular and molecular targets for the immunotherapy of hepatocellular carcinoma. Mol Cell Biochem 437:13–36
Mantovani A (2009) The yin-yang of tumor-associated neutrophils. Cancer Cell 16:173–174
Sarrouilhe D, Clarhaut J, Defamie N, Mesnil M (2015) Serotonin and cancer: what is the link? Curr Mol Med 15:62–77
Nieswandt B, Hafner M, Echtenacher B, Mannel DN (1999) Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res 59:1295–1300
Rungsakulkij N, Mingphruedhi S, Suragul W, Tangtawee P, Muangkaew P, Aeesoa S (2018) Platelet-to-lymphocyte ratio and large tumor size predict microvascular invasion after resection for hepatocellular carcinoma. Asian Pac J Cancer Prev 19:3435–3441
Zheng J, Seier K, Gonen M et al (2017) Utility of serum inflammatory markers for predicting microvascular invasion and survival for patients with hepatocellular carcinoma. Ann Surg Oncol 24:3706–3714
Yu Y, Song J, Zhang R et al (2017) Preoperative neutrophil-to-lymphocyte ratio and tumor-related factors to predict microvascular invasion in patients with hepatocellular carcinoma. Oncotarget 8:79722–79730
Wu J, Chen M, Liang C, Su W (2017) Prognostic value of the pretreatment neutrophil-to-lymphocyte ratio in cervical cancer: a meta-analysis and systematic review. Oncotarget 8:13400–13412
Liu C, Jia BS, Zou BW et al (2017) Neutrophil-to-lymphocyte and aspartate-to-alanine aminotransferase ratios predict hepatocellular carcinoma prognosis after transarterial embolization. Medicine (Baltimore) 96:e8512
Xiao WK, Chen D, Li SQ, Fu SJ, Peng BG, Liang LJ (2014) Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. BMC Cancer 14:117
Xue TC, Jia QA, Ge NL et al (2015) The platelet-to-lymphocyte ratio predicts poor survival in patients with huge hepatocellular carcinoma that received transarterial chemoembolization. Tumour Biol 36:6045–6051
Sullivan KM, Groeschl RT, Turaga KK et al (2014) Neutrophil-to-lymphocyte ratio as a predictor of outcomes for patients with hepatocellular carcinoma: a Western perspective. J Surg Oncol 109:95–97
Nicolini D, Agostini A, Montalti R et al (2017) Radiological response and inflammation scores predict tumour recurrence in patients treated with transarterial chemoembolization before liver transplantation. World J Gastroenterol 23:3690–3701
Liu C, Li L, Lu WS et al (2018) A novel combined systemic inflammation-based score can predict survival of intermediate-to-advanced hepatocellular carcinoma patients undergoing transarterial chemoembolization. BMC Cancer 18:216
Kwon HC, Kim SH, Oh SY et al (2012) Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers 17:216–222
Wang D, Wu M, Feng FZ et al (2013) Pretreatment neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios do not predict survival in patients with cervical cancer treated with neoadjuvant chemotherapy and radical hysterectomy. Chin Med J (Engl) 126:1464–1468
van der Woude LL, Gorris MAJ, Halilovic A, Figdor CG, de Vries IJM (2017) Migrating into the tumor: a roadmap for T cells. Trends Cancer 3:797–808
Motomura T, Shirabe K, Mano Y et al (2013) Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol 58:58–64
Pufnock JS, Rothstein JL (2009) Oncoprotein signaling mediates tumor-specific inflammation and enhances tumor progression. J Immunol 182:5498–5506
Shimada M, Rikimaru T, Hamatsu T et al (2001) The role of macroscopic classification in nodular-type hepatocellular carcinoma. Am J Surg 182:177–182
Harding JJ, El Dika I, Abou-Alfa GK (2016) Immunotherapy in hepatocellular carcinoma: primed to make a difference? Cancer 122:367–377
Xue TC, Jia QA, Ge NL, Chen Y, Zhang BH, Ye SL (2015) Imbalance in systemic inflammation and immune response following transarterial chemoembolization potentially increases metastatic risk in huge hepatocellular carcinoma. Tumour Biol 36:8797–8803
Li F, Guo Z, Lizee G, Yu H, Wang H, Si T (2014) Clinical prognostic value of CD4+CD25+FOXP3+regulatory T cells in peripheral blood of Barcelona Clinic Liver Cancer (BCLC) stage B hepatocellular carcinoma patients. Clin Chem Lab Med 52:1357–1365
Mizukoshi E, Nakamoto Y, Arai K et al (2011) Comparative analysis of various tumor-associated antigen-specific T-cell responses in patients with hepatocellular carcinoma. Hepatology 53:1206–1216
Jia ZZ, Jiang GM, Feng YL (2011) Serum HIF-1alpha and VEGF levels pre- and post-TACE in patients with primary liver cancer. Chin Med Sci J 26:158–162
Warburg O, Gawehn K, Geissler AW (1960) The transformation of embryonal metabolism in cancer metabolism. Z Naturforsch B 15B:378–379
Semaan A, Dietrich D, Bergheim D et al (2017) CXCL12 expression and PD-L1 expression serve as prognostic biomarkers in HCC and are induced by hypoxia. Virchows Arch 470:185–196
Zhou ZQ, Tong DN, Guan J et al (2016) Follicular helper T cell exhaustion induced by PD-L1 expression in hepatocellular carcinoma results in impaired cytokine expression and B cell help, and is associated with advanced tumor stages. Am J Transl Res 8:2926–2936
Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331:1565–1570
Acknowledgments
We thank Luzie Dömel for her support and Claus Peter Nowak, M.Sc., for providing statistical advice. Dr. Savic is a participant in the BIH-Charité Junior Clinician Scientist Program funded by the Charité – Universiätsmedizin Berlin and the Berlin Institute of Health.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Julius Chapiro.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Claus Peter Nowak, M.Sc., from the Institute of Biometry and Clinical Epidemiology, Charité Berlin, kindly provided statistical advice for this manuscript.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at a single institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 25 kb)
Rights and permissions
About this article
Cite this article
Schobert, I.T., Savic, L.J., Chapiro, J. et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of tumor response in hepatocellular carcinoma after DEB-TACE. Eur Radiol 30, 5663–5673 (2020). https://doi.org/10.1007/s00330-020-06931-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-06931-5