Abstract
Objectives
To characterize the chest computed tomography (CT) findings of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) according to clinical severity. We compared the CT features of common cases and severe cases, symptomatic patients and asymptomatic patients, and febrile and afebrile patients.
Methods
This was a retrospective analysis of the clinical and thoracic CT features of 120 consecutive patients with confirmed SARS-CoV-2 pneumonia admitted to a tertiary university hospital between January 10 and February 10, 2020, in Wuhan city, China.
Results
On admission, the patients generally complained of fever, cough, shortness of breath, and myalgia or fatigue, with diarrhea often present in severe cases. Severe patients were 20 years older on average and had comorbidities and an elevated lactate dehydrogenase (LDH) level. There were no differences in the CT findings between asymptomatic and symptomatic common type patients or between afebrile and febrile patients, defined according to Chinese National Health Commission guidelines.
Conclusions
The clinical and CT features at admission may enable clinicians to promptly evaluate the prognosis of patients with SARS-CoV-2 pneumonia. Clinicians should be aware that clinically silent cases may present with CT features similar to those of symptomatic common patients.
Key Points
• The clinical features and predominant patterns of abnormalities on CT for asymptomatic, typic common, and severe cases were summarized. These findings may help clinicians to identify severe patients quickly at admission.
• Clinicians should be cautious that CT findings of afebrile/asymptomatic patients are not better than the findings of other types of patients. These patients should also be quarantined.
• The use of chest CT as the main screening method in epidemic areas is recommended.
Similar content being viewed by others
Introduction
An outbreak of novel coronavirus–infected (SARS-CoV-2) pneumonia in Wuhan City, China [1] has caused a global health emergency [2]. The number of patients is rapidly increasing out of China. The continuous expansion of COVID-19 has created a pandemic [3].
SARS-CoV-2 belongs to the new coronavirus of the genus β. The pneumonia that this coronavirus causes resembles severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). However, the genome of SARS-CoV-2 is significantly different from that of SARS-CoV and MERS-CoV [4], and thus, this virus may cause different clinical presentations, including different chest CT findings [5,6,7]. To date, the severity of the illness has been reported to be milder than SARS with a mortality rate ranging from 4.3 [8] to 11% [9]. The incubation time (the time interval between initial contact with an infectious agent and appearance of the first sign or symptom of disease) tends to be longer (median 3 days, range 0–24 days) than that of SARS [10, 11]. The imaging findings of SARS-CoV-2 pneumonia [12,13,14,15,16,17,18,19,20] overlap with those of other viral pneumonia [21, 22]. However, COVID-19 might still be classified by some distinguishable features. The longer incubation time and relatively mild symptoms at presentation may allow this disease to more easily spread from humans to humans due to little concern. Prompt screening for the early identification and isolation of the patients are of particular importance. Therefore, chest CT scans might be ideal for this purpose.
Other recently published studies have shown that descriptions of CT abnormalities of SARS-CoV-2 pneumonia as a function of clinical severity are still limited. Little is known about afebrile pneumonia cases. Less is known about asymptomatic pneumonia cases. Therefore, we attempt to systematically assess these issues in a retrospective cohort of 120 consecutive patients with positive chest CT findings at admission.
Methods
Study design and participants
This was a single-center, retrospective, observational study conducted at a tertiary hospital. A cohort of 120 consecutive patients with confirmed SARS-CoV-2 infection from January 1 to February 10, 2020, at the Renmin Hospital of Wuhan University were included. Cases were initially identified by abnormal findings on chest CT scan. And the patients were also diagnosed based on the WHO interim guidance [23]; that is, specimens from the respiratory tract of patients were collected for SARS-CoV-2 testing by RT-PCR. The definition of severe type in this study included all severe type and critical ill type diseases defined by the National Guidelines of China [24]. Severe COVID-19 was designated when the patients met one of the following criteria: (1) respiratory distress with a breathing rate ≥ 30/min; (2) pulse oximeter oxygen saturation ≤ 93% at rest; (3) oxygenation index (artery partial pressure of oxygen/inspired oxygen fraction, PaO2/FiO2) ≤ 300 mmHg; (4) respiratory failure requiring mechanical ventilation; (5) shock; and (6) combined with other organ failure requiring intensive care unit (ICU) monitoring and treatment. Patients were then confirmed as SARS-CoV-2 pneumonia by test of reverse transcriptase–polymerase chain reaction (RT-PCR) for SARS-CoV-2 RNA from paired nasopharyngeal swabs [10, 25], following the recommendation by the National Institute for Viral Disease Control and Prevention of China [26]. We additionally categorized two mild types among the common types: (1) asymptomatic type: patients with no symptoms; and (2) afebrile type: patients have symptoms but a body temperature < 37.3 °C. Since all patients included in this cohort had positive chest CT findings, we defined them as patients with SARS-CoV-2 pneumonia instead of COVID-19.
Data collection
Epidemiological, clinical, laboratory, and radiological characteristics and outcomes data were obtained with standardized data collection form created by EpiData software (version 3.1). Clinical data were double-entered by two medical residents (H.K.J. and H.J.L.). The final data were obtained by consensus when there was a discrepancy.
The following epidemiological and clinical data were collected, including age, sex, Hunan seafood market exposure history, smoking status, the final clinical diagnosis, comorbidities (diabetes, hypertension, cardiovascular disease, chronic obstructive pulmonary disease, malignancy, and chronic liver disease) and clinical symptoms (fever, highest body temperature (°C), cough, dyspnea, myalgia or fatigue, headache, sneezing, rhinorrhea, sputum production, hemoptysis, gastrointestinal discomfort; loss of appetite and diarrhea), and survival status. The following laboratory tests were collected: white blood cell count, neutrophil count, lymphocyte count, and lactate dehydrogenase (LDH, U/L) at admission, where other test results such as liver function test and renal function test were not collected because all results were found to be within normal range during the quick review.
Image acquisition and interpretation
All CT examinations for the screening of SARS-CoV-2 pneumonia were performed with two GE scanners (64-section Optima CT680 and 16-section BrightSpeed) without the use of contrast material. The main scanning protocol was as follows: tube voltage, 120 kVp; tube current modulation, 120 mA–380 mA; detector configuration, 64 × 0.625 mm or 16 × 0.625 mm; rotation time, 0.5–0.7 s; slice thickness, 5 mm; and pitch, 0.984. Reconstruction kernel was lung with a thickness and an interval of 0.625 mm. All images were viewed in both lung (width, 1200 HU; level, − 700 HU) and mediastinal (width, 350 HU; level, 40 HU) settings. Two radiologists (Z.R. and F.L.L.) with 6–7 years of experiences who were blinded to the other clinical information reviewed the chest CT scans independently and in random order, and then reached a decision by consensus. For interpretation disagreement between the two primary radiologists, a senior radiologist (O.Y.H.Q.) with 20 years of experience provided a final decision.
The images were interpreted using the lung window setting. The CT images were assessed, following a standardized protocol, for the presence and distribution of the following abnormalities: (a) ground-glass opacities (GGO, defined as hazy areas of increased attenuation without obscuration of the underlying vascular markings); (b) nodules (centrilobular, perilymphatic, or random in distribution); (c) linear densities (interlobular septal thickening, intralobular septal line, parenchymal bands); (d) crazy paving; (e) consolidations (parenchymal opacities obscuring underlying vessels); (f) architectural distortion, or traction bronchiectasis; (g) pleural effusion; (h) lymphadenopathy (defined as lymph node with a short-axis dimension of > 1.0 cm); (i) air bronchogram; (j) tree-in-bud sign (defined as multiple areas of centrilobular nodules with a linear branching pattern); and (k) white lung (defined as diffuse consolidations in a large area of the lung that appear like the lung is turning white on CT imaging). The overall anatomic distribution (subsegmental, segmental, lobar), zonal predominance (upper, middle, lower lung; central, middle, or peripheral location), and extent (focal, multifocal, and diffuse) of the lesions were also recorded. The predominant patterns of abnormality on high-resolution CT were classified into consolidation, GGOs, reticulation, and mixed patterns. A mixed pattern can be described as presence of crazy paving and of air bronchogram. Each of the five lung lobes was assessed for degree of involvement and classified as follows: none (0%) corresponded to a lobe score of 0, mild (1–25%) corresponded to a lobe score of 1, moderate (26–50%) corresponded to a lobe score of 2, severe (51–75%) corresponded to a lobe score of 3, and critical (76–100%) corresponded to a lobe score of 4. The “total severity score” was calculated by summing the scores of all five lobes (range of possible scores, 0–20).
Statistical analysis
Continuous variables were reported with means and were compared with Student t test or analysis of variance (ANOVA); categorical variables were expressed as percentage and compared by χ2 test or Fisher’s exact test. Inter-observer agreement for the radiographical abnormalities was evaluated and expressed with the Kappa statistic. The agreement was classified as follows: excellent, Kappa > 0.80; good, Kappa = 0.61–0.80; moderate, Kappa = 0.41–0.60; fair, Kappa = 0.21–0.40; and poor, < 0.20. p values ≤ 0.05 (2-sided probability) were considered statistically significant. All analyses were conducted using SPSS 23.0 software (IBM Corp).
Results
Clinical and laboratory findings
Patients’ characteristics according to disease severity are presented in Table 1. There were 43 men and 77 women included in this study. Among the 120 patients, 16 were totally asymptomatic, 74 had typical common cases, and 30 were severe. All 6 current smokers are found in severe cases, and all 7 deaths were severe cases. Severe cases were on average 21 years older than common cases (61.2 vs. 40.2 years old, p < 0.001). Comorbidities were more frequent in severe patients than in patients with common case severity (73% vs. 11%, p < 0.001). Sneezing, sputum production, and diarrhea were commonly seen among severe patients but not among common ones. Hemoptysis was rarely found for all patients. Regarding laboratory test results, common patients had lower neutrophil counts (mean 1.6 × 109/L) and lower LDH levels (mean 200.8 U/L, normal range < 250 U/L) than the severe patients (mean 342.8 U/L). However, there was no evidence of difference in sex distribution, Huanan seafood market exposure history, and white blood cell count or lymphocyte count.
Radiologic findings
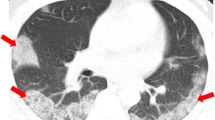
At admission, the most frequent CT finding was GGOs, which were present in 87% and 97% of common and severe type patients, respectively (p = 0.181). A comparison between common and severe type imaging features is presented in Table 2. Consolidation, air bronchogram, white lung appearance, and pleural effusion were more frequently seen in severe patients (p < 0.001), with crazy-paving patterns, linear densities, bronchiectasis, nodules, and tree-in-bud signs also being more frequent. Regarding the repartition, bilateral diffuse involvement was more frequent in severe type, with all 5 lobes’ involvement more frequently seen and higher lobar score severity. Common cases showed more frequent peripheral and lower lung predominance, with limited, focal, or multifocal subsegmental extent. Illustrative cases are presented Fig. 1.
(a) Unenhanced axial CT images of an afebrile 37-year-old male doctor with a history of exposure to confirmed SARS-CoV-2 patients. (a1) Patchy ground-glass opacities (GGOs) in the left upper lobe. (a2) Small GGO nodule in the contralateral lower lobe. (A3) Enlarged image of the right lower lobe. (b) Unenhanced axial CT images of an afebrile 28-year-old female with a history of exposure to confirmed SARS-CoV-2 patients presenting with a mild sore throat. (b1) A rounded, ground-glass nodular opacity (GGO) is seen in a subpleural location in the right lower lobe. (b2) Another focal GGO is seen in a subpleural location, in the posterobasal segment of the left lower lobe. (c) Unenhanced axial CT images of a 27-year-old male doctor with a history of exposure to confirmed SARS-CoV-2 patients, initially presenting with fever (39 °C), non-productive cough, dyspnea, and myalgia (c1) who progressed to a severe case requiring oxygen supplementation (c2). (c1) Multifocal, limited GGO is seen in the peripheral zone of both lungs. (c2) Six days later, while oxygen supplementation has been instore, diffuse, bilateral, and ill-defined GGO has developed. Superimposed linear consolidations can be observed, consistent with areas of organizing pneumonia. (d) Unenhanced axial CT images of a 52-year-old male doctor with asthma and exposure to confirmed SARS-CoV-2 patients, initially presenting with fever (39 °C), non-productive cough, dyspnea, and myalgia who rapidly progressed to a severe form requiring mechanical ventilation. (d1) Multifocal, limited GGO in the periphery of both lungs, predominantly affecting left lung. (d2) Two days later, focal GGO has increased in size and density, and new diffuse ill-defined GGO has developed. (d3) After 4 days of mask oxygen supplementation, disease progressed further, with more patchy consolidations and linear densities observed in nearly all lung zones except the anterior part of both lungs. (e) Unenhanced axial CT images of a 57-year-old male with an exposure history initially presenting with fever (38 °C), non-productive cough, dyspnea, myalgia, and headache, being treated for hypertension for 12 years. Diffuse consolidation with air bronchograms is seen in both lungs, with relative sparing of peri-hilar and anterior lung areas, extending from the lung apices to the lung bases. These findings are consistent with a “white lung” appearance
A comparison of the CT features between totally asymptomatic and symptomatic common cases is presented in Table 3. There were no significant differences in individual signs, patterns, zonal predominance, or extent of CT abnormalities. Similarly, there were no significant differences in CT features between febrile and afebrile common patients, as shown in Table 4.
The Kappa statistic for inter-observer agreement was evaluated as good for most CT findings, and detailed information is demonstrated in Supplementary Table.
Prognosis factors for patients with SARS-CoV-2
Table 5 shows significant associations of select patient characteristics with severe disease after a multivariate model analysis. Overall, statistically significant associations with the risk of severe disease were observed among those with older age, those with symptoms of dyspnea, and those chest CT findings of crazy-paving patterns (OR = 15.3, 95% CI = 2.6–89.5) and air bronchogram (OR = 41.8, 95% CI = 5.9–298.4).
Discussion
In this study, we reported the clinical characteristics and chest CT findings at presentation for all types of SARS-CoV-2 pneumonia severity. We found that severe type patients showed differ rent CT features as compared with common type individuals, but we also report that asymptomatic patients may present with the same CT findings as presented by symptomatic common type patients. Our findings compensated for the knowledge gaps in previous reports on this newly emerging disease.
Consistent with previous studies [8, 9, 11], the main symptoms at presentation were fever, dry cough, myalgia or fatigue, and dyspnea. Some atypical symptoms such as headache, sneezing, and diarrhea were only found in severe patients.
In line with other studies [12,13,14,15,16,17,18,19], the predominant patterns of abnormalities on CT for our 120 patients were GGOs with a peripheral distribution and bilateral, multifocal lower lung involvement. Compared with those of common cases, some CT features were more common in severe cases, such as crazy-paving patterns, bronchiectasis, hilar or mediastinal lymph node enlargement, white lung, air bronchogram, and pleural effusion. We also found that severe cases usually presented as consolidation larger than segmental consolidation, and only severe cases showed an overall lobar or diffuse pneumonia distribution pattern, which indicated that the affected area or volume could also be an important determinant for the disease severity of SARS-CoV-2 pneumonia. However, these patterns also often overlap because CT manifestations are dynamic [15, 16], and depend on various factors, such as disease severity, the evolution of the disease course [14, 15, 20], treatment [12], comorbidity, and complications. Usually, as the disease progressed, the range of GGO patches and consolidation increased. Afterwards, condition would improve, GGO and consolidation disappeared, and fibrous stripes (reticulation) may appear [20].
We did not find any appreciable CT imaging difference between common patients with or without fever, or even between those with or without any symptoms. Ai et al [27] suggested that with RT-PCR results as a reference, the sensitivity, specificity, and accuracy of chest CT in indicating SARS-CoV-2 infection were 97% (95% CI 95–98%, 580/601 patients), 25% (95% CI 22–30%, 105/413 patients), and 68% (95% CI 65–70%, 685/1014 patients), respectively. Thus, quick recognition of the radiological manifestations and a prompt evaluation of the patient’s exposure history is imperative in the early detection and assessment of the severity of SARS-CoV-2 pneumonia. Numerically, more consolidation and reticulation patterns were found on the CT image of silent and afebrile patients than those of typic patients. This indicated a later detection of mild disease. The transmissibility for these silent cases needs to be further assessed. In addition, during the long incubation period (0–24 days, mostly 3 days) [11], patients might also be unwitting vehicles of disease spread.
Our study has several limitations. As with any retrospective study, the probability of selection bias and the availability of needed data is a concern. RT-PCR-based tests were used to confirm the infection status, which may minimize the misclassification error. The findings of the statistical tests should be interpreted with caution even though our cohort is relatively large. However, the strong associations we found can still can alert physicians to potentially poor prognoses in some cases. The subjectivity of image interpretation may also introduce measurement errors, but these errors were likely to be random. We took a systematic approach that allowed us to obtain valuable statistics and to quantify the imaging changes, thus minimizing the subjectivity. Moreover, we followed a standardize protocol; thus, inter-observer agreement was good for the evaluation of most radiographical abnormalities. As previously described, our cohort may be unique in that the patients admitted were first screened by chest CT, and were admitted from nearby communities to a general hospital rather than a specialized hospital of infectious disease. Thus, these patients are more representative of the community population.
In conclusion, the typical CT features we described and some radiological findings (e.g. consolidation, air bronchogram, and diffuse extent.), together with some clinic-biological characteristics of severity (older age, comorbidities, diarrhea, and elevated LDH level), may help clinicians in assessing the severity of SARS-CoV-2 pneumonia quickly at admission of patients. The non-difference in CT findings between asymptomatic or nonfebrile patients and symptomatic common patients highlight the importance of CT screening for silent patients for the timely diagnosis and transmission prevention.
Abbreviations
- ANOVA:
-
Analysis of variance
- CT:
-
Computed tomography
- GGO:
-
Ground-glass opacities
- ICU:
-
Intensive care unit
- LDH:
-
Lactate dehydrogenase
- MERS:
-
Middle East respiratory syndrome
- MERS-CoV:
-
Middle East Respiratory Syndrome Novel Coronavirus
- RT-PCR:
-
Reverse transcriptase–polymerase chain reaction
- SARS:
-
Severe acute respiratory syndrome
- SARS-CoV:
-
Severe acute respiratory syndrome novel coronavirus
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
References
Zhu N, Zhang D, Wang W et al (2020) A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382:727–733
World Health Organization (2020) Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). WHO, Geneva, Switzerland
World Health Organization (2020) WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. https://www.who.int/dg/speeches/detail/whodirector-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
Zhou P, Yang XL, Wang XG et al (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 1038:020–2012
Wong KT, Antonio GE, Hui DS et al (2003) Severe acute respiratory syndrome: radiographic appearances and pattern of progression in 138 patients. Radiology 228:401–406
Paul NS, Roberts H, Butany J et al (2004) Radiologic pattern of disease in patients with severe acute respiratory syndrome: the Toronto experience. Radiographics 24:553–563
Assiri A, Al-Tawfiq JA, Al-Rabeeah AA et al (2013) Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis 13:752–761
Wang D, Hu B, Hu C et al (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323(11):1061–1069
Chen N, Zhou M, Dong X et al (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 6736:30211–30217
Li Q, Guan X, Wu P et al (2020) Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 382:1199–1207
Guan W-j, Ni Z-y, Hu Y et al (2020) Clinical characteristics of 2019 novel coronavirus infection in China. medRxiv. https://doi.org/10.1101/2020.02.06.20020974
Duan Y-n, Qin J (2020) Pre- and posttreatment chest CT findings: 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology 295(1):21
Pan Y, Guan H (2020) Imaging changes in patients with 2019-nCov. Eur Radiol. https://doi.org/10.1007/s00330-020-06713-z
Pan Y, Guan H, Zhou S et al (2020) Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. https://doi.org/10.1007/s00330-020-06731-x
Shi H, Han X, Zheng C (2020) Evolution of CT manifestations in a patient recovered from 2019 novel coronavirus (2019-nCoV) pneumonia in Wuhan, China. Radiology 295(1):20
Song F, Shi N, Shan F et al (2020) Emerging coronavirus 2019-nCoV pneumonia. Radiology 295(1):210–217
Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J (2020) Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. https://doi.org/10.1148/radiol.2020200343
Chung M, Bernheim A, Mei X et al (2020) CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology 295(1):202–207
Lei J, Li J, Li X, Qi X (2020) CT imaging of the 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology 295(1):18
Pan F, Ye T, Sun P et al (2020) Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. https://doi.org/10.1148/radiol.2020200370
Franquet T (2011) Imaging of pulmonary viral pneumonia. Radiology 260:18–39
Koo HJ, Lim S, Choe J, Choi SH, Sung H, Do KH (2018) Radiographic and CT features of viral pneumonia. Radiographics 38:719–739
World Health Organization (2020) Clinical management of severe acute respiratory infection when Novel coronavirus (nCoV) infection is suspected: interim guidance. https://apps.who.int/iris/handle/10665/330893
Pneumonia diagnosis and treatment of novel coronavirus infection (trial version 5). National health commission of the People’s Republic of China http://www.nhc.gov.cn/yzygj/s7653p/202002/3b09b894ac9b4204a79db5b8912d4440/files/7260301a393845fc87fcf6dd52965ecb.pdf
National Health Commission of the People’s Republic of China. Prevention of 2019-nCoV infected pneumonia, version 2. website: http://www.nhc.gov.cn/jkj/s3577/202001/c67cfe29ecf1470e8c7fc47d3b751e88.shtml. Accessed on 8 Feb 2020
National Institute for Viral Disease Control and Prevention of China. Specific primers and probes for detection 2019 novel coronavirus. website: http://ivdc.chinacdc.cn/kyjz/202001/t20200121_211337.html Accessed on 8 Feb 2020
Ai T, Yang Z, Hou H et al (2020) Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. https://doi.org/10.1148/radiol.2020200642
Acknowledgements
The survey was conducted by a group of clinicians who are taking care of patients with 2019-nCoV pneumonia at the frontline. We would like to thank this research team and our patients for their heroic fighting against the disease.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Zhenming Fu.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study because this is a retrospective observational study.
Ethical approval
This retrospective study was approved by the institutional review board of Renmin Hospital of Wuhan University (No. WDRY2020-K007).
Methodology
• Retrospective
• Observational
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary table
The Kappa test of recognizing the CT change of the early and advanced stage SARS-CoV-2 patients (DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Zhang, R., Ouyang, H., Fu, L. et al. CT features of SARS-CoV-2 pneumonia according to clinical presentation: a retrospective analysis of 120 consecutive patients from Wuhan city. Eur Radiol 30, 4417–4426 (2020). https://doi.org/10.1007/s00330-020-06854-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-06854-1