Abstract
Objectives
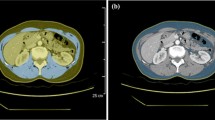
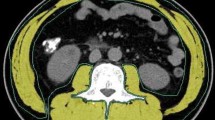
The loss of skeletal muscle mass is widely considered a predictor of poor survival and toxicity in breast cancer patients. The aim of this study is to evaluate if there is pectoralis muscle area (PMA) variation, reflecting loss of skeletal muscle mass, on consecutive MRI examinations after neoadjuvant chemotherapy.
Methods
The retrospective study protocol was approved by our institutional review board. A total of n = 110 consecutive patients (mean age 56 ± 11 years) who were treated with neoadjuvant chemotherapy (NAC) for histologically proven primary breast cancer between January 2017 and January 2019 and in whom tumor response was checked with standard breast MRI were included. Two radiologists calculated the pectoralis muscle cross-sectional area before and after NAC.
Results
Time between the MRI examinations, before starting NAC and after completing NAC, was 166.8 ± 50 days. PMA calculated pre-NAC (8.14 cm2) was larger than PMA calculated post-NAC (7.03 cm2) (p < 0.001). According to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria, there were no significant differences between responders (complete or partial response) and non-responders (p = 0.362). The multivariate regression analysis did not show any significant relationships between ΔPMA and age, time between MRI exams, estrogen and progesterone receptor status, human epidermal growth factor receptor status (HER-2), Ki-67 expression, lymph node status, RECIST criteria, histological type, average lesion size, molecular categories, and grade. Inter-reader (k = 0.72) and intra-reader agreement (0.69 and 0.71) in PMA assessment were good.
Conclusions
Pectoralis muscle mass varies in breast cancer patients undergoing NAC and this difference can be estimated directly on standard breast MRI.
Key Points
• Pectoralis muscle area variation reflects loss of skeletal muscle mass.
• Pectoralis muscle area on MRI is reduced after NAC.
• Pectoralis muscle mass loss seems independent from other variables.



Similar content being viewed by others
Abbreviations
- FLASH:
-
Fast low-angle shot
- HER-2:
-
Human epidermal growth factor receptor status
- MRI:
-
Magnetic resonance imaging
- NAC:
-
Neoadjuvant chemotherapy
- PMA:
-
Pectoralis muscle area
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- TIRM:
-
Turbo inversion recovery magnitude
References
Caan BJ, Cespedes Feliciano EM, Prado CM et al (2018) Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol 4:798–804
Deluche E, Leobon S, Desport JC, Venat-Bouvet L, Usseglio J, Tubiana-Mathieu N (2018) Impact of body composition on outcome in patients with early breast cancer. Support Care Cancer 26:861–868
Klassen O, Schmidt ME, Ulrich CM et al (2017) Muscle strength in breast cancer patients receiving different treatment regimes. J Cachexia Sarcopenia Muscle 8:305–316
Villaseñor A, Ballard-Barbash R, Baumgartner K et al (2012) Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL study. J Cancer Surviv 6:398–406
Mazzuca F, Onesti CE, Roberto M et al (2018) Lean body mass wasting and toxicity in early breast cancer patients receiving anthracyclines. Oncotarget 9:25714–25722
Kaufmann M, von Minckwitz G, Mamounas EP et al (2012) Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol 19:1508–1516
Sardanelli F, Boetes C, Borisch B et al (2010) Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur J Cancer 46:1296–1316
Mann RM, Balleyguier C, Baltzer PA et al (2015) Breast MRI: EUSOBI recommendations for women’s information. Eur Radiol 25:3669–3678
Rossi F, Valdora F, Barabino E, Calabrese M, Tagliafico AS (2019) Muscle mass estimation on breast magnetic resonance imaging in breast cancer patients: comparison between psoas muscle area on computer tomography and pectoralis muscle area on MRI. Eur Radiol 29:494–500
Rossi F, Valdora F, Bignotti B, Torri L, Succio G, Tagliafico AS (2019) Evaluation of body computed tomography-determined sarcopenia in breast cancer patients and clinical outcomes: a systematic review. Cancer Treat Res Commun 21:100154
Seo A, Hwang JM, Lee JM, Jung TD (2019) Changes in pectoral muscle volume during subacute period after radiation therapy for breast cancer: a retrospective up to 4-year follow-up study. Sci Rep 9:7038
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Londero V, Bazzocchi M, Del Frate C et al (2004) Locally advanced breast cancer: comparison of mammography, sonography and MR imaging in evaluation of residual disease in women receiving neoadjuvant chemotherapy. Eur Radiol 14:1371–1379
Del Fabbro E, Parsons H, Warneke CL et al (2012) The relationship between body composition and response to neoadjuvant chemotherapy in women with operable breast cancer. Oncologist 17:1240–1245
Rier HN, Jager A, Sleijfer S, van Rosmalen J, Kock MCJM, Levin MD (2018) Changes in body composition and muscle attenuation during taxane-based chemotherapy in patients with metastatic breast cancer. Breast Cancer Res Treat 168:95–105
Prado CM, Baracos VE, McCargar LJ et al (2009) Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 15:2920–2926
Iwase T, Sangai T, Nagashima T et al (2016) Impact of body fat distribution on neoadjuvant chemotherapy outcomes in advanced breast cancer patients. Cancer Med 5:41–48
Strigel RM, Rollenhagen J, Burnside ES et al (2017) Screening breast MRI outcomes in routine clinical practice: comparison to BI-RADS benchmarks. Acad Radiol 24:411–417
Saslow D, Boetes C, Burke W et al (2007) American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 57:75–89
Saini A, Faulkner S, Al-Shanti N, Stewart C (2009) Powerful signals for weak muscles. Ageing Res Rev 8:251–267
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Alberto Stefano Tagliafico.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Prof. Alberto Stefano Tagliafico kindly provided statistical advice for this manuscript.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective
• Cross-sectional study
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rossi, F., Torri, L., Lambertini, M. et al. Muscle mass loss after neoadjuvant chemotherapy in breast cancer: estimation on breast magnetic resonance imaging using pectoralis muscle area. Eur Radiol 30, 4234–4241 (2020). https://doi.org/10.1007/s00330-020-06799-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-06799-5