Abstract
Objectives
R2* cardiac magnetic resonance (CMR) allows the non-invasive measurement of myocardial iron. We calibrated cardiac R2* values against myocardial tissue–measured iron concentration by using a segmental approach and we assessed the iron distribution.
Methods
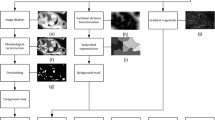
Five hearts of thalassemia patients were donated after death/transplantation to the CoreLab of the Myocardial Iron Overload in Thalassemia Network. A multislice multiecho R2* approach was adopted. After CMR, used as guidance, the heart was cut in three short-axis slices and each slice was cut into different equiangular segments according to AHA segmentation and differentiated into endocardial and epicardial layers. Tissue iron concentration was measured by atomic absorption spectrometer technique.
Results
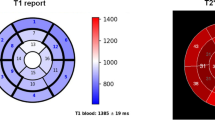
Fifty-five samples were used since only for two hearts all the 16 samples were analyzed. Mean iron concentration was 4.71 ± 4.67 mg/g dw. Segmental iron levels ranged from 0.24 to 13.78 mg/g dw. The coefficient of variability of iron for myocardial segments ranged from 8.08 to 24.54% (mean 13.49 ± 6.93%). Iron concentration was significantly higher in the epicardial than in the endocardial layer (5.99 ± 6.01 vs 4.84 ± 4.87 mg/g dw; p = 0.042). Four different circumferential regions (anterior, septal, inferior, and lateral) were defined. A circumferential heterogeneity was noted, with more iron in the anterior region, followed by the inferior region. The direct nonlinear fitting of R2* and [Fe] data led to the calibration curve: [Fe] = 0.0022 ∙ (R2*-ROI)1.462 (R-square = 0.956).
Conclusions
Our data further validate R2* CMR using a segmental approach as a sensitive and early technique for quantifying iron distribution in the current clinical practice.
Key Points
• Calibration in humans for cardiovascular magnetic resonance R2* against myocardial iron concentration was provided.
• A circumferential heterogeneity in cardiac iron distribution was detected: more iron was observed in the anterior region, followed by the inferior region. This finding corroborates the use of a segmental T2* CMR approach in the clinical practice to detect a heterogeneous iron distribution.
• The comparison between the cardiac T2* values obtained with the region-based and the pixel-wise approaches showed a significant correlation and no significant difference but, in presence of significant iron load, the region-based approach resulted in significantly higher T2* values.





Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- CMR:
-
Cardiovascular magnetic resonance
- CoV:
-
Coefficient of variation
- EMB:
-
Endomyocardial biopsy
- LV:
-
Left ventricle
- MIO:
-
Myocardial iron overload
- MIOT:
-
Myocardial Iron Overload in Thalassemia
- MRI:
-
Magnetic resonance imaging
- PW:
-
Pixel-wise
- RB:
-
Region-based
- ROI:
-
Region of interest
- SD:
-
Standard deviation
- TE:
-
Echo times
- TM:
-
Thalassemia major
References
Hentze MW, Muckenthaler MU, Andrews NC (2004) Balancing acts: molecular control of mammalian iron metabolism. Cell 117:285–297
Davis BA, Porter JB (2000) Long-term outcome of continuous 24-hour deferoxamine infusion via indwelling intravenous catheters in high-risk beta-thalassemia. Blood 95:1229–1236
Meloni A, Positano V, Ruffo GB et al (2015) Improvement of heart iron with preserved patterns of iron store by CMR-guided chelation therapy. Eur Heart J Cardiovasc Imaging 16:325–334
Cogliandro T, Derchi G, Mancuso L et al (2008) Guideline recommendations for heart complications in thalassemia major. J Cardiovasc Med (Hagerstown) 9:515–525
Mavrogeni S, Pepe A, Lombardi M (2011) Evaluation of myocardial iron overload using cardiovascular magnetic resonance imaging. Hellenic J Cardiol 52:385–390
Olson LJ, Edwards WD, Holmes DR Jr, Miller FA Jr, Nordstrom LA, Baldus WP (1989) Endomyocardial biopsy in hemochromatosis: clinicopathologic correlates in six cases. J Am Coll Cardiol 13:116–120
Leone O, Veinot JP, Angelini A et al (2012) 2011 consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc Pathol 21:245–274
House MJ, Fleming AJ, de Jonge MD et al (2014) Mapping iron in human heart tissue with synchrotron x-ray fluorescence microscopy and cardiovascular magnetic resonance. J Cardiovasc Magn Reson 16:80
Gossuin Y, Roch A, Muller RN, Gillis P (2000) Relaxation induced by ferritin and ferritin-like magnetic particles: the role of proton exchange. Magn Reson Med 43:237–243
Carpenter JP, He T, Kirk P et al (2011) On T2* magnetic resonance and cardiac iron. Circulation 123:1519–1528
Meloni A, Rienhoff HY Jr, Jones A, Pepe A, Lombardi M, Wood JC (2014) Cardiac R2* values are independent of the image analysis approach employed. Magn Reson Med 72:485–491
Meloni A, Ramazzotti A, Positano V et al (2009) Evaluation of a web-based network for reproducible T2* MRI assessment of iron overload in thalassemia. Int J Med Inform 78:503–512
Ramazzotti A, Pepe A, Positano V et al (2009) Multicenter validation of the magnetic resonance t2* technique for segmental and global quantification of myocardial iron. J Magn Reson Imaging 30:62–68
Pepe A, Positano V, Santarelli F et al (2006) Multislice multiecho T2* cardiovascular magnetic resonance for detection of the heterogeneous distribution of myocardial iron overload. J Magn Reson Imaging 23:662–668
Pepe A, Lombardi M, Positano V et al (2006) Evaluation of the efficacy of oral deferiprone in beta-thalassemia major by multislice multiecho T2*. Eur J Haematol 76:183–192
Positano V, Pepe A, Santarelli MF et al (2007) Standardized T2* map of normal human heart in vivo to correct T2* segmental artefacts. NMR Biomed 20:578–590
Cerqueira MD, Weissman NJ, Dilsizian V et al (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 105:539–542
Positano V, Meloni A, Santarelli MF et al (2015) Fast generation of T2* maps in the entire range of clinical interest: application to thalassemia major patients. Comput Biol Med 56:200–210
Beilby JP, Prins AW, Swanson NR (1999) Determination of hepatic iron concentration in fresh and paraffin-embedded tissue. Clin Chem 45:573–574
Pepe A, Meloni A, Rossi G et al (2018) Prediction of cardiac complications for thalassemia major in the widespread cardiac magnetic resonance era: a prospective multicentre study by a multi-parametric approach. Eur Heart J Cardiovasc Imaging 19:299–309
Olson LJ, Edwards WD, McCall JT, Ilstrup DM, Gersh BJ (1987) Cardiac iron deposition in idiopathic hemochromatosis: histologic and analytic assessment of 14 hearts from autopsy. J Am Coll Cardiol 10:1239–1243
Ghugre NR, Enriquez CM, Gonzalez I, Nelson MD Jr, Coates TD, Wood JC (2006) MRI detects myocardial iron in the human heart. Magn Reson Med 56:681–686
Meloni A, Positano V, Pepe A et al (2010) Preferential patterns of myocardial iron overload by multislice multiecho T*2 CMR in thalassemia major patients. Magn Reson Med 64:211–219
Anderson LJ, Holden S, Davis B et al (2001) Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J 22:2171–2179
Wood JC, Enriquez C, Ghugre N et al (2005) MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood 106:1460–1465
Casale M, Meloni A, Filosa A et al (2015) Multiparametric cardiac magnetic resonance survey in children with thalassemia major: a multicenter study. Circ Cardiovasc Imaging 8:e003230
Positano V, Pepe A, Santarelli MF et al (2009) Multislice multiecho T2* cardiac magnetic resonance for the detection of heterogeneous myocardial iron distribution in thalassaemia patients. NMR Biomed 22:707–715
Hankins JS, McCarville MB, Loeffler RB et al (2009) R2* magnetic resonance imaging of the liver in patients with iron overload. Blood 113:4853–4855
Raya JG, Dietrich O, Horng A, Weber J, Reiser MF, Glaser C (2010) T2 measurement in articular cartilage: impact of the fitting method on accuracy and precision at low SNR. Magn Reson Med 63:181–193
Feng Y, He T, Gatehouse PD et al (2013) Improved MRI R(2) * relaxometry of iron-loaded liver with noise correction. Magn Reson Med 70:1765–1774
Wang C, Zhang X, Liu X et al (2018) Improved liver R2* mapping by pixel-wise curve fitting with adaptive neighborhood regularization. Magn Reson Med 80:792–801
Funding
The MIOT project receives “no-profit support” from industrial sponsorships (Chiesi Farmaceutici S.p.A. and ApoPharma Inc.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Alessia Pepe.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from the parents of all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Prospective
• Observational
• Multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meloni, A., Maggio, A., Positano, V. et al. CMR for myocardial iron overload quantification: calibration curve from the MIOT Network. Eur Radiol 30, 3217–3225 (2020). https://doi.org/10.1007/s00330-020-06668-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-06668-1