Abstract
Objectives
To explore the value of multiparametric MRI combined with FDG-PET/CT to identify well-responding rectal cancer patients before the start of neoadjuvant chemoradiation.
Methods
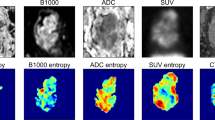
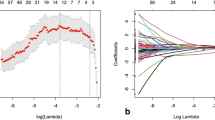
Sixty-one locally advanced rectal cancer patients who underwent a baseline FDG-PET/CT and MRI (T2W + DWI) and received long-course neoadjuvant chemoradiotherapy were retrospectively analysed. Tumours were delineated on MRI and PET/CT from which the following quantitative parameters were calculated: T2W volume and entropy, ADC mean and entropy, CT density (mean-HU), SUV maximum and mean, metabolic tumour volume (MTV42%) and total lesion glycolysis (TLG). These features, together with sex, age, mrTN-stage (“baseline parameters”) and the CRT-surgery interval were analysed using multivariable stepwise logistic regression. Outcome was a good (TRG 1–2) versus poor histopathological response. Performance (AUC) to predict response was compared for different combinations of baseline ± quantitative imaging parameters and performance in an ‘independent’ dataset was estimated using bootstrapped leave-one-out cross-validation (LOOCV).
Results
The optimal multivariable prediction model consisted of a combination of baseline + quantitative imaging parameters and included mrT-stage (OR 0.004, p < 0.001), T2W-signal entropy (OR 7.81, p = 0.0079) and T2W volume (OR 1.028, p = 0.0389) as the selected predictors. AUC in the study dataset was 0.88 and 0.83 after LOOCV. No PET/CT features were selected as predictors.
Conclusions
A multivariable model incorporating mrT-stage and quantitative parameters from baseline MRI can aid in identifying well-responding patients before the start of treatment. Addition of FDG-PET/CT is not beneficial.
Key Points
• A multivariable model incorporating the mrT-stage and quantitative features derived from baseline MRI can aid in identifying well-responding patients before the start of neoadjuvant chemoradiotherapy.
• mrT-stage was the strongest predictor in the model and was complemented by the tumour volume and signal entropy calculated from T2W-MRI.
• Adding quantitative features derived from pre-treatment PET/CT or DWI did not contribute to the model’s predictive performance.


Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AIC:
-
Akaike Information Criterion
- AUC:
-
Area under the receiver operating characteristic (ROC) curve
- CRT:
-
Chemoradiotherapy
- CT:
-
Computed tomography
- DWI:
-
Diffusion-weighted imaging
- FDG:
-
Fluorodeoxyglucose; 2-deoxy-2-[18F]fluoro-D-glucose; 18F-FDG
- Gy:
-
Gray
- HU:
-
Hounsfield unit
- LARC:
-
Locally advanced rectal cancer
- LOOCV:
-
Leave-one-out cross-validation
- MRI:
-
Magnetic resonance imaging
- MTV:
-
Metabolic tumour volume
- OR:
-
Odds ratio
- PET/CT:
-
Positron-emission tomography/computed tomography
- SUV:
-
Standardised uptake value
- T2W:
-
T2-weighted
- TLG:
-
Total lesion glycolysis
- TRG:
-
Tumour regression grade
- W&W:
-
Watch-and-wait
References
Maas M, Nelemans PJ, Valentini V et al (2010) Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 11:835–844
Habr-Gama A, Perez RO, Wynn G, Marks J, Kessler H, Gama-Rodrigues J (2010) Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: characterization of clinical and endoscopic findings for standardization. Dis Colon Rectum 53:1692–1698
Paun BC, Cassie S, MacLean AR, Dixon E, Buie WD (2010) Postoperative complications following surgery for rectal cancer. Ann Surg 251:807–818
Hendren SK, O’Connor BI, Liu M et al (2005) Prevalence of male and female sexual dysfunction is high following surgery for rectal cancer. Ann Surg 242:212–223
van der Valk MJM, Hilling DE, Bastiaannet E et al (2018) Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet 391:2537–2545
Maas M, Beets-Tan RGH, Lambregts DMJ et al (2011) Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol 29:4633–4640
Habr-Gama A, Gama-Rodrigues J, São Julião GP et al (2014) Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys 88:822–828
Smith JD, Ruby JA, Goodman KA et al (2012) Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg 256:965–972
Appelt AL, Pløen J, Harling H et al (2015) High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol 16:919–927
Rombouts AJM, Al-Najami I, Abbott NL et al (2017) Can we Save the rectum by watchful waiting or TransAnal microsurgery following (chemo) Radiotherapy versus Total mesorectal excision for early REctal Cancer (STAR-TREC study)?: protocol for a multicentre, randomised feasibility study. BMJ Open 7:e019474
Van Stiphout RGPM, Valentini V, Buijsen J et al (2014) Nomogram predicting response after chemoradiotherapy in rectal cancer using sequential PETCT imaging: a multicentric prospective study with external validation. Radiother Oncol 113:215–222
Janssen MHM, Öllers MC, Van Stiphout RGPM et al (2012) PET-based treatment response evaluation in rectal cancer: prediction and validation. Int J Radiat Oncol Biol Phys 82:871–876
Maffione AM, Marzola MC, Capirci C, Colletti PM, Rubello D (2015) Value of 18 F-FDG PET for predicting response to neoadjuvant therapy in rectal cancer: systematic review and meta-analysis. AJR Am J Roentgenol 204:1261–1268
Cliffe H, Patel C, Prestwich R, Scarsbrook A (2017) Radiotherapy response evaluation using FDG PET-CT—established and emerging applications. Br J Radiol 90:20160764
Joye I, Debucquoy A, Deroose CM et al (2017) Quantitative imaging outperforms molecular markers when predicting response to chemoradiotherapy for rectal cancer. Radiother Oncol 124:104–109
Schurink NW, Lambregts DM, Beets-Tan RG (2019) Diffusion-weighted imaging in rectal cancer: current applications and future perspectives. Br J Radiol 92:20180655
Joye I, Deroose CM, Vandecaveye V, Haustermans K (2014) The role of diffusion-weighted MRI and 18F-FDG PET/CT in the prediction of pathologic complete response after radiochemotherapy for rectal cancer: a systematic review. Radiother Oncol 113:158–165
Mahadevan LS, Zhong J, Venkatesulu BP et al (2018) Imaging predictors of treatment outcomes in rectal cancer: an overview. Crit Rev Oncol Hematol 129:153–162
Meng X, Huang Z, Wang R, Yu J (2014) Prediction of response to preoperative chemoradiotherapy in patients with locally advanced rectal cancer. Biosci Trends 8:11–23
Giannini V, Mazzetti S, Bertotto I et al (2019) Predicting locally advanced rectal cancer response to neoadjuvant therapy with 18 F-FDG PET and MRI radiomics features. Eur J Nucl Med Mol Imaging 46:878–888
Van Griethuysen JJM, Fedorov A, Parmar C et al (2017) Computational radiomics system to decode the radiographic phenotype. Cancer Res 77:e104–e107
Erdi YE, Mawlawi O, Larson SM et al (1997) Segmentation of lung lesion volume by adaptive positron emission tomography image thresholding. Cancer 80:2505–2509
Miccò M, Vargas HA, Burger IA et al (2014) Combined pre-treatment MRI and 18F-FDG PET/CT parameters as prognostic biomarkers in patients with cervical cancer. Eur J Radiol 83:1169–1176
Ueno Y, Lisbona R, Tamada T, Alaref A, Sugimura K, Reinhold C (2017) Comparison of FDG PET metabolic tumour volume versus ADC histogram: prognostic value of tumour treatment response and survival in patients with locally advanced uterine cervical cancer. Br J Radiol 90:20170035
Shu Z, Fang S, Ye Q et al (2019) Prediction of efficacy of neoadjuvant chemoradiotherapy for rectal cancer: the value of texture analysis of magnetic resonance images. Abdom Radiol (NY) 21:1051–1058
Greenbaum A, Martin DR, Bocklage T, Lee JH, Ness SA, Rajput A (2019) Tumor heterogeneity as a predictor of response to neoadjuvant chemotherapy in locally advanced rectal cancer. Clin Colorectal Cancer 18:102–109
Bozkaya Y, Özdemir NY, Erdem GU et al (2018) Clinical predictive factors associated with pathologic complete response in locally advanced rectal cancer. J Oncol Sci 4:5–10
Deantonio L, Caroli A, Puta E et al (2018) Does baseline [18F] FDG-PET/CT correlate with tumor staging, response after neoadjuvant chemoradiotherapy, and prognosis in patients with rectal cancer? Radiat Oncol 13:211
Traverso A, Wee L, Dekker A, Gillies R (2018) Repeatability and reproducibility of radiomic features: a systematic review. Int J Radiat Oncol Biol Phys 102:1143–1158
Mandard A-M, Dalibard F, Mandard J-C et al (1994) Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 73:2680–2686
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19:716–723
Burnham KP, Anderson DR (2004) Multimodel Inference. Sociol Methods Res 33:261–304
Akgun E, Caliskan C, Bozbiyik O et al (2018) Randomized clinical trial of short or long interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg 105:1417–1425
Joye I, Debucquoy A, Fieuws S et al (2016) Can clinical factors be used as a selection tool for an organ-preserving strategy in rectal cancer? Acta Oncol 55:1047–1052
Al-Sukhni E, Attwood K, Mattson DM, Gabriel E, Nurkin SJ (2016) Predictors of pathologic complete response following neoadjuvant chemoradiotherapy for rectal cancer. Ann Surg Oncol 23:1177–1186
Lahaye MJ, Engelen SME, Nelemans PJ et al (2005) Imaging for predicting the risk factors—the circumferential resection margin and nodal disease—of local recurrence in rectal cancer: a meta-analysis. Semin Ultrasound CT MR 26:259–268
Gröne J, Loch FN, Taupitz M, Schmidt C, Kreis ME (2018) Accuracy of various lymph node staging criteria in rectal cancer with magnetic resonance imaging. J Gastrointest Surg 22:146–153
Francois Y, Nemoz CJ, Baulieux J et al (1999) Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol 17:2396–2396
Kalady MF, de Campos-Lobato LF, Stocchi L et al (2009) Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Trans Meet Am Surg Assoc 127:213–220
Foster JD, Jones EL, Falk S, Cooper EJ, Francis NK (2013) Timing of surgery after long-course neoadjuvant chemoradiotherapy for rectal cancer: a systematic review of the literature. Dis Colon Rectum 56:921–930
Probst CP, Becerra AZ, Aquina CT et al (2015) Extended intervals after neoadjuvant therapy in locally advanced rectal cancer: the key to improved tumor response and potential organ preservation. J Am Coll Surg 221:430–440
Petrelli F, Sgroi G, Sarti E, Barni S (2016) Increasing the interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer. Ann Surg 263:458–464
Meng Y, Zhang C, Zou S et al (2018) MRI texture analysis in predicting treatment response to neoadjuvant chemoradiotherapy in rectal cancer. Oncotarget 9:11999–12008
De Cecco CN, Rengo M, Meinel FG et al (2015) Texture analysis as imaging biomarker of tumoral response to neoadjuvant chemoradiotherapy in rectal cancer patients studied with 3-T magnetic resonance. Invest Radiol 50:239–245
Martens MH, Van Heeswijk MM, Van Den Broek JJ et al (2015) Prospective, multicenter validation study of magnetic resonance volumetry for response assessment after preoperative chemoradiation in rectal cancer: can the results in the literature be reproduced? Int J Radiat Oncol Biol Phys 93:1005–1014
Lambregts DMJ, Rao S-X, Sassen S et al (2015) MRI and diffusion-weighted MRI volumetry for identification of complete tumor responders after preoperative chemoradiotherapy in patients with rectal cancer. Ann Surg 262:1034–1039
Curvo-Semedo L, Lambregts DMJ, Maas M et al (2011) Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy—conventional MR volumetry versus diffusion-weighted MR imaging. Radiology 260:734–743
Quaia E, Gennari AG, Ricciardi MC et al (2016) Value of percent change in tumoral volume measured at T2 -weighted and diffusion-weighted MRI to identify responders after neoadjuvant chemoradiation therapy in patients with locally advanced rectal carcinoma. J Magn Reson Imaging 44:1415–1424
Ha HI, Kim AY, Yu CS, Park SH, Ha HK (2013) Locally advanced rectal cancer: diffusion-weighted MR tumour volumetry and the apparent diffusion coefficient for evaluating complete remission after preoperative chemoradiation therapy. Eur Radiol 23:3345–3353
Young HK, Dae YK, Tae HK et al (2005) Usefulness of magnetic resonance volumetric evaluation in predicting response to preoperative concurrent chemoradiotherapy in patients with resectable rectal cancer. Int J Radiat Oncol Biol Phys 62:761–768
Okuno T, Kawai K, Koyama K et al (2018) Value of FDG–PET/CT volumetry after chemoradiotherapy in rectal cancer. Dis Colon Rectum 61:320–327
Dos Anjos DA, Perez RO, Habr-Gama A et al (2016) Semiquantitative volumetry by sequential PET/CT may improve prediction of complete response to neoadjuvant chemoradiation in patients with distal rectal cancer. Dis Colon Rectum 59:805–812
Park J, Chang KJ, Seo YS et al (2014) Tumor SUVmax normalized to liver uptake on 18 F-FDG PET/CT predicts the pathologic complete response after neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Nucl Med Mol Imaging 48:295–302
Funding
This study has received funding by the Dutch Cancer Society (project number 10138).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Doenja MJ Lambregts.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors, Mr. Sander Roberti, has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Boards (retrospective analysis of prospectively obtained observational data).
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in:
Cusomano (2018) Radiol Med
Van Stiphout (2014) Radiother Oncol
Janssen (2012) Int J Rad Oncol Biol Phys
Van Stiphout (2011) Radiother Oncol
Janssen (2010) Int J Rad Oncol Biol Phys
Janssen (2010) Radiother Oncol
Lambregts (2011) Ann Surg Oncol
Lambregts (2015) Ann Surg
Martens (2015) Int J Radiat Oncol Biol Phys
Lambregts (2018) Dis Colon rectum
Methodology
• Retrospective
• Diagnostic or prognostic study
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Table 1
(DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Schurink, N.W., Min, L.A., Berbee, M. et al. Value of combined multiparametric MRI and FDG-PET/CT to identify well-responding rectal cancer patients before the start of neoadjuvant chemoradiation. Eur Radiol 30, 2945–2954 (2020). https://doi.org/10.1007/s00330-019-06638-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06638-2