Abstract
Objectives
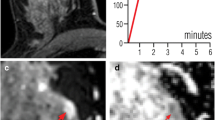
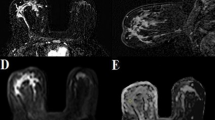
This study aimed to develop a tool for the classification of masses in breast MRI, based on ultrafast TWIST-VIBE Dixon (TVD) dynamic sequences combined with DWI. TVD sequences allow to abbreviate breast MRI protocols, but provide kinetic information only on the contrast wash-in, and because of the lack of the wash-out kinetics, their diagnostic value might be hampered. A special focus of this study was thus to maintain high diagnostic accuracy in lesion classification.
Materials and methods
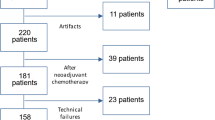
Sixty-one patients who received breast MRI between 02/2014 and 04/2015 were included, with 83 reported lesions (60 malignant). Our institute’s standard breast MRI protocol was complemented by an ultrafast TVD sequence. ADC and peak enhancement of the TVD sequences were integrated into a generalised linear model (GLM) for malignancy prediction. For comparison, a second GLM was calculated using ADC and conventional DCE curve type. The resulting GLMs were evaluated for standard diagnostic parameters. For easy application of the GLMs, nomograms were created.
Results
The GLM based on peak enhancement of the TVD and ADC was as equally accurate as the GLM based on conventional DCE and ADC, with no significant differences (sensitivity, 93.3%/93.3%; specificity, 91.3%/87.0%; PPV, 96.6%/94.9%; NPV, 84.0%/83.3%; all, p ≥ 0.315).
Conclusions
This study presents a method to integrate ultrafast TVD sequences into a breast MRI protocol, allowing a reduction of the examination time while maintaining diagnostic accuracy. A GLM based on the combination of TVD-derived peak enhancement and ADC provides high diagnostic accuracy, and can be easily applied using a nomogram.
Key Points
• Ultrafast TWIST-VIBE Dixon sequence protocols in combination with diffusion-weighted imaging allow to shorten breast MRI examinations, while diagnostic accuracy is maintained.
• Integrating peak enhancement from the TWIST-VIBE Dixon sequence and the apparent diffusion coefficient into a generalised linear model provides a comprehensible image evaluation approach.
• This approach is further facilitated by nomograms.




Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AIC:
-
Akaike information criteria
- AUC:
-
Area under the curve
- DCE:
-
Dynamic contrast enhancement/enhanced
- DWI:
-
Diffusion-weighted imaging
- GLM:
-
Generalised linear model
- LOOCV:
-
Leave-one-out cross-validation
- MRI:
-
Magnetic resonance imaging
- MS:
-
Maximum slope
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region of interest
- SPAIR:
-
Spectral attenuated inversion recovery
- STIR:
-
Short tau inversion recovery
- TTE:
-
Time-to-enhancement
- TVD:
-
TWIST-VIBE Dixon
- TWIST:
-
Time-resolved angiography with stochastic trajectories
- VIBE:
-
Volumetric interpolated breath-hold examination
References
Turnbull LW (2009) Dynamic contrast-enhanced MRI in the diagnosis and management of breast cancer. NMR Biomed 22:28–39
Xu T, Zhang L, Xu H et al (2017) Prediction of low-risk breast cancer using quantitative DCE-MRI and its pathological basis. Oncotarget 8:114360–114370
Baltzer A, Dietzel M, Kaiser CG, Baltzer PA (2016) Combined reading of contrast enhanced and diffusion weighted magnetic resonance imaging by using a simple sum score. Eur Radiol 26:884–891
Pinker K, Moy L, Sutton EJ et al (2018) Diffusion-weighted imaging with apparent diffusion coefficient mapping for breast cancer detection as a stand-alone parameter: comparison with dynamic contrast-enhanced and multiparametric magnetic resonance imaging. Invest Radiol 53:587–595
Pinker-Domenig K, Bogner W, Gruber S et al (2012) High resolution MRI of the breast at 3 T: which BI-RADS® descriptors are most strongly associated with the diagnosis of breast cancer? Eur Radiol 22:322–330
Mann RM, Kuhl CK, Kinkel K, Boetes C (2008) Breast MRI:guidelines from the European Society of Breast Imaging. Eur Radiol 18:1307–1318
Mango VL, Morris EA, David Dershaw D et al (2015) Abbreviated protocol for breast MRI: are multiple sequences needed for cancer detection? Eur J Radiol 84:65–70
Grimm LJ, Soo MS, Yoon S, Kim C, Ghate SV, Johnson KS (2015) Abbreviated screening protocol for breast MRI: a feasibility study. Acad Radiol 22:1157–1162
Harvey SC, Di Carlo PA, Lee B, Obadina E, Sippo D, Mullen L (2016) An abbreviated protocol for high-risk screening breast MRI saves time and resources. J Am Coll Radiol 13:R74–R80
Heacock L, Melsaether AN, Heller SL et al (2016) Evaluation of a known breast cancer using an abbreviated breast MRI protocol:correlation of imaging characteristics and pathology with lesion detection and conspicuity. Eur J Radiol 85:815–823
Moschetta M, Telegrafo M, Rella L, Stabile Ianora AA, Angelelli G (2016) Abbreviated combined MR protocol: a new faster strategy for characterizing breast lesions. Clin Breast Cancer 16:207–211
Kuhl CK, Schrading S, Strobel K et al (2014) Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J Clin Oncol 32:2304–2310
Fischer U, Korthauer A, Baum F et al (2012) Short first-pass MRI of the breast. Acta Radiol 53:267–269
Schrading S, Kuhl CK (2008) Mammographic, US, and MR imaging phenotypes of familial breast cancer. Radiology 246:58–70
Dietzel M, Baltzer P, Vag T et al (2011) Magnetic resonance mammography in small vs. advanced breast lesions - systematic comparison reveals significant impact of lesion size on diagnostic accuracy in 936 histologically verified breast lesions. Rofo 183:126–135
Mann RM, Mus RD, Van Zelst J et al (2014) A novel approach to contrast-enhanced breast magnetic resonance imaging for screening:high-resolution ultrafast dynamic imaging. Invest Radiol 49:579–585
Tsao J, Kozerke S (2012) MRI temporal acceleration techniques. J Magn Reson Imaging 36:543–560
Platel B, Mus R, Welte T et al (2014) Automated characterization of breast lesions imaged with an ultrafast DCE-MR protocol. IEEE Trans Med Imaging 33:225–232
Le Y, Kipfer H, Majidi S et al (2013) Application of time-resolved angiography with stochastic trajectories (TWIST)-Dixon in dynamic contrast-enhanced (DCE) breast MRI. J Magn Reson Imaging 38:1033–1042
Le Y, Kipfer HD, Nickel DM et al (2016) Initial experience of applying TWIST-Dixon with flexible view sharing in breast DCEMRI. Clin Breast Cancer 16:202–206
Clauser P, Pinker K, Helbich TH et al (2014) Fat saturation in dynamic breast MRI at 3 tesla: is the Dixon technique superior to spectral fat saturation? A visual grading characteristics study. Eur Radiol 24:2213–2219
Le Y, Kroeker R, Kipfer HD, Lin C (2012) Development and evaluation of TWIST Dixon for dynamic contrast-enhanced (DCE) MRI with improved acquisition efficiency and fat suppression. J Magn Reson Imaging 36:483–491
Herrmann K-H, Baltzer PA, Dietzel M et al (2011) Resolving arterial phase and temporal enhancement characteristics in DCEMRM at high spatial resolution with TWIST acquisition. J Magn Reson Imaging 34:973–982
Eby PR, DeMartini WB, Gutierrez RL et al (2009) Characteristics of probably benign breast MRI lesions. AJR Am J Roentgenol 193:861–867
Dietzel M, Ellmann S, Schulz-Wendtland R et al (2019) Breast MRI in the era of diffusion weighted imaging: do we still need signal-intensity time curves? Eur Radiol. https://doi.org/10.1007/s00330-019-06346-x
Horos - Free DICOM Medical Image Viewer. Available via https://horosproject.org/. Accessed 15 Mar 2017
DCE Tool Plugin. Available via http://kyungs.bol.ucla.edu/software/DCE_tool/DCE_tool.html. Accessed 1 Jun 2018
Bickel H, Pinker K, Polanec S et al (2017) Diffusion-weighted imaging of breast lesions: region-of-interest placement and different ADC parameters influence apparent diffusion coefficient values. Eur Radiol 27:1883–1892
Kuhl CK, Mielcareck P, Klaschik S et al (1999) Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions? Radiology 211:101–110
Morris EAE, Comstock CC, Lee CC, et al (2013) ACR BI-RADS® magnetic resonance imaging. In: ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System, 5th ed. American College of Radiology, Reston, VA, VA
Moser K, Patnick J, Beral V (2007) Do women know that the risk of breast cancer increases with age? Br J Gen Pract 57:404–406
Liberman L, Mason G, Morris EA, Dershaw DD (2006) Does size matter? Positive predictive value of MRI-detected breast lesions as a function of lesion size. AJR Am J Roentgenol 186:426–430
RStudio Team (2015) RStudio: integrated development for R. Available via https://rstudio.com.
Kuhn M (2016) caret: classification and regression training. R package version 6.0-71. Available via https://cran.r-project.org/web/packages/caret/index.html.
Cawley GC, Talbot NLC (2004) Fast exact leave-one-out cross validation of sparse least-squares support vector machines. Neural Netw 17:1467–1475
Doerfler R (2009) The lost art of nomography. UMAP J 30:457–493
Kul S, Cansu A, Alhan E et al (2011) Contribution of diffusion weighted imaging to dynamic contrast-enhanced MRI in the characterization of breast tumors. AJR Am J Roentgenol 196:210–217
Pediconi F, Miglio E, Telesca M et al (2012) Effect of preoperative breast magnetic resonance imaging on surgical decision making and cancer recurrence rates. Invest Radiol 47:128–135
Belli P, Bufi E, Bonatesta A et al (2016) Unenhanced breast magnetic resonance imaging: detection of breast cancer. Eur Rev Med Pharmacol Sci 20:4220–4229
Le Bihan D, Poupon C, Amadon A, Lethimonnier F (2006) Artifacts and pitfalls in diffusion MRI. J Magn Reson Imaging 24:478–488
Saadatmand S, Tilanus-Linthorst MMA, Rutgers EJT et al (2013) Cost-effectiveness of screening women with familial risk for breast cancer with magnetic resonance imaging. J Natl Cancer Inst 105:1314–1321
Pataky R, Armstrong L, Chia S et al (2013) Cost-effectiveness of MRI for breast cancer screening in BRCA1/2 mutation carriers. BMC Cancer 13:339
Taneja C, Edelsberg J, Weycker D et al (2009) Cost effectiveness of breast cancer screening with contrast-enhanced MRI in high-risk women. J Am Coll Radiol 6:171–179
Grimes DA (2008) The nomogram epidemic: resurgence of a medical relic. Ann Intern Med 149:273–275
Balachandran VP, Gonen M, Smith JJ, DeMatteo RP (2015) Nomograms in oncology: more than meets the eye. Lancet Oncol 16:e173–e180
Ogura A, Hayakawa K, Miyati T, Maeda F (2011) Imaging parameter effects in apparent diffusion coefficient determination of magnetic resonance imaging. Eur J Radiol 77:185–188
Camps-Herrero J (2019) Diffusion-weighted imaging of the breast:current status as an imaging biomarker and future role. BJR Open 1:20180049. https://doi.org/10.1259/bjro.20180049
Yuen S, Yamada K, Goto M et al (2009) Microperfusion-induced elevation of ADC is suppressed after contrast in breast carcinoma. J Magn Reson Imaging 29:1080–1084
Amornsiripanitch N, Bickelhaupt S, Shin HJ et al (2019) Diffusion weighted MRI for unenhanced breast cancer screening. Radiology:182789. https://doi.org/10.1148/radiol.2019182789
Tamura T, Murakami S, Naito K et al (2014) Investigation of the optimal b-value to detect breast tumors with diffusion weighted imaging by 1.5-T MRI. Cancer Imaging:14. https://doi.org/10.1186/1470-7330-14-11
Han X, Li J, Wang X (2017) Comparison and optimization of 3.0 T breast images quality of diffusion-weighted imaging with multiple B-values. Acad Radiol 24:418–425
Alexander DLJ, Tropsha A, Winkler DA (2015) Beware of R(2):simple, unambiguous assessment of the prediction accuracy of QSAR and QSPR models. J Chem Inf Model 55:1316–1322
Rao RB, Fung G, Rosales R (2008) On the dangers of cross-validation. An experimental evaluation. Proc 2008 SIAMInt ConfData Min. https://doi.org/10.1137/1.9781611972788.54
Kinkel K, Hylton NM (2001) Challenges to interpretation of breast MRI. J Magn Reson Imaging 13:821–829
Tozaki M (2004) Interpretation of breast MRI: correlation of kinetic and morphological parameters with pathological findings. Magn Reson Med Sci 3:189–197
Erguvan-Dogan B, Whitman GJ, Kushwaha AC et al (2006) BIRADS-MRI: a primer. AJR Am J Roentgenol 187:W152–W160
Sardanelli F, Boetes C, Borisch B et al (2010) Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur J Cancer 46:1296–1316
Vreemann S, Rodriguez-Ruiz A, Nickel D et al (2017) Compressed sensing for breast MRI: resolving the trade-off between spatial and temporal resolution. Invest Radiol 52:574–582
Grimm LJ, Anderson AL, Baker JA et al (2015) Interobserver variability between breast imagers using the fifth edition of the BIRADS MRI lexicon. AJR Am J Roentgenol 204:1120–1124
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Stephan Ellmann.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: Evelyn Wenkel, Rolf Janka, and Michael Uder are members of the Siemens Speakers’ Bureau. Elisabeth Weiland is a Siemens Healthcare GmbH employee. All other authors have no potential conflict of interest to declare.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained, and the need for informed consent was waived because of the retrospective nature of this study.
Methodology
• Retrospective
• Diagnostic or prognostic study
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 220 kb)
Rights and permissions
About this article
Cite this article
Peter, S.C., Wenkel, E., Weiland, E. et al. Combination of an ultrafast TWIST-VIBE Dixon sequence protocol and diffusion-weighted imaging into an accurate easily applicable classification tool for masses in breast MRI. Eur Radiol 30, 2761–2772 (2020). https://doi.org/10.1007/s00330-019-06608-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06608-8