Abstract
Objectives
The objective of this study was to evaluate the intra-individual, longitudinal consistency of iodine measurements regarding the vascular and renal blood pool in patients that underwent repetitive spectral detector computed tomography (SDCT) examinations to evaluate their utility for oncologic imaging.
Methods
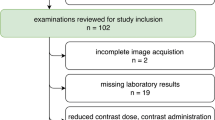
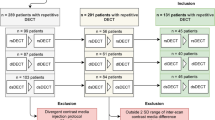
Seventy-nine patients with two (n = 53) or three (n = 26) clinically indicated biphasic SDCT scans of the abdomen were retrospectively included. ROI-based measurements of Hounsfield unit (HU) attenuation in conventional images and iodine concentration were performed by an experienced radiologist in the following regions (two ROIs each): abdominal aorta, vena cava inferior, portal vein, and renal cortices. Modified variation coefficients (MVCs) were computed to assess intra-individual longitudinal between the different time points.
Results
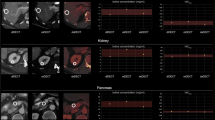
Variation of HU attenuation and iodine concentration measurements was significantly lower in the venous than in the arterial phase images (attenuation/iodine concentration: arterial − 4.2/− 3.9, venous 0.4/1.0; p ≤ 0.05). Regarding attenuation in conventional images of the arterial phase, the median MVC was − 1.8 (− 20.5–21.3) % within the aorta and − 6.5 (− 44.0–25.0) % within the renal cortex while in the portal venous phase, it was 0.62 (− 11.1–11.7) % and − 1.6 (− 16.2–10.6) %, respectively. Regarding iodine concentration, MVC for arterial phase was − 2.5 (− 22.9–28.4) % within the aorta and − 5.8 (− 55.9–29.6) % within the renal cortex. The referring MVCs of the portal venous phase were − 0.7 (− 17.9–16.9) % and − 2.6 (− 17.6–12.5) %.
Conclusions
Intra-individual iodine quantification of the vascular and cortical renal blood pool at different time points works most accurately in venous phase images whereas measurements conducted in arterial phase images underlay greater variability.
Key Points
• There is an intra-individual, physiological variation in iodine map measurements from dual-energy computed tomography.
• This variation is smaller in venous phase examinations compared with arterial phase and therefore venous phase images should be preferred to minimize this intra-individual variation.
• Care has to be taken, when considering iodine measurements for clinical decision-making, particularly in the context of oncologic initial or follow-up imaging.






Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variances
- AP:
-
Anterior-posterior
- CI:
-
Conventional images
- CT:
-
Computed tomography
- CTDIvol:
-
Volumetric computed tomography dose index
- DE :
-
Effective diameter
- DECT:
-
Dual-energy computed tomography
- HU:
-
Hounsfield units
- IC:
-
Iodine concentration
- ICC:
-
Intraclass correlation coefficient
- IM:
-
Iodine maps
- LAT:
-
Lateral
- MVC:
-
Modified variation coefficient
- RADS:
-
Reporting and data storage system
- RECIST:
-
Response evaluation criteria in solid tumors
- RIS:
-
Radiological information system
- ROI:
-
Region of interest
- SDCT:
-
Spectral detector computed tomography
References
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Chung K, Jacobs C, Scholten ET et al (2017) Lung-RADS category 4X: does it improve prediction of malignancy in subsolid nodules? Radiology 284(1):264–271
Schima W, Heiken J (2018) LI-RADS v2017 for liver nodules: how we read and report. Cancer Imaging 18
Seymour L, Bogaerts J, Perrone A et al (2017) iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 18(3):e143–e152
Gordic S, Puippe GD, Krauss B et al (2016) Correlation between dual-energy and perfusion CT in patients with hepatocellular carcinoma. Radiology 280(1):78–87
Yang C-B, Zhang S, Jia Y-J et al (2017) Dual energy spectral CT imaging for the evaluation of small hepatocellular carcinoma microvascular invasion. Eur J Radiol 95:222–227
Mileto A, Marin D, Alfaro-Cordoba M et al (2014) Iodine quantification to distinguish clear cell from papillary renal cell carcinoma at dual-energy multidetector CT: a multireader diagnostic performance study. Radiology 273(3):813–820
Graser A, Becker CR, Staehler M et al (2010) Single-phase dual-energy CT allows for characterization of renal masses as benign or malignant. Invest Radiol 45(7):399–405
Kawamoto S, Fuld MK, Laheru D, Huang P, Fishman EK (2018) Assessment of iodine uptake by pancreatic cancer following chemotherapy using dual-energy CT. Abdom Radiol (NY) 43(2):445–456
Noda Y, Goshima S, Miyoshi T et al (2018) Assessing chemotherapeutic response in pancreatic ductal adenocarcinoma: histogram analysis of iodine concentration and CT number in single-source dual-energy CT. AJR Am J Roentgenol. https://doi.org/10.2214/AJR.18.19791
Pelgrim GJ, van Hamersvelt RW, Willemink MJ et al (2017) Accuracy of iodine quantification using dual energy CT in latest generation dual source and dual layer CT. Eur Radiol 27(9):3904–3912
Jacobsen MC, Schellingerhout D, Wood CA et al (2018) Intermanufacturer comparison of dual-energy CT iodine quantification and monochromatic attenuation: a phantom study. Radiology 287(1):224–234
Sauter AP, Kopp FK, Münzel D et al (2018) Accuracy of iodine quantification in dual-layer spectral CT: influence of iterative reconstruction, patient habitus and tube parameters. Eur J Radiol 102:83–88
Hua C-H, Shapira N, Merchant TE, Klahr P, Yagil Y (2018) Accuracy of electron density, effective atomic number, and iodine concentration determination with a dual-layer dual-energy computed tomography system. Med Phys 45(6):2486–2497
Große Hokamp N, Abdullayev N, Persigehl T et al (2018) Precision and reliability of liver iodine quantification from spectral detector CT: evidence from phantom and patient data. Eur Radiol. https://doi.org/10.1007/s00330-018-5744-0
Carlström M, Wilcox CS, Arendshorst WJ (2015) Renal autoregulation in health and disease. Physiol Rev 95(2):405–511
Boone JM, Strauss KJ, Cody DD et al American Association of Physicists in Medicine (AAPM). Size-specific dose estimates (SSDE) in pediatric and adult body CT examinations: report of AAPM Task Group 204. Available via: https://www.aapm.org/pubs/reports/RPT_204.pdf. Accessed 24 Apr 2019
Große Hokamp N, Höink AJ, Doerner J et al (2018) Assessment of arterially hyper-enhancing liver lesions using virtual monoenergetic images from spectral detector CT: phantom and patient experience. Abdom Radiol (NY) 43(8):2066–2074
Reed GF, Lynn F, Meade BD (2002) Use of coefficient of variation in assessing variability of quantitative assays. Clin Diagn Lab Immunol 9(6):1235–1239
Fleiss JL, Cohen J (2016) The equivalence of weighted kappa and the intraclass correlation coefficient as measures of reliability. Educ Psychol Meas 33(3):613–619
Thaiss WM, Haberland U, Kaufmann S et al (2016) Iodine concentration as a perfusion surrogate marker in oncology: further elucidation of the underlying mechanisms using volume perfusion CT with 80 kVp. Eur Radiol 26(9):2929–2936
Uhrig M, Simons D, Bonekamp D, Schlemmer H-P (2017) Improved detection of melanoma metastases by iodine maps from dual energy CT. Eur J Radiol 90:27–33
Muenzel D, Lo GC, Yu HS et al (2017) Material density iodine images in dual-energy CT: detection and characterization of hypervascular liver lesions compared to magnetic resonance imaging. Eur J Radiol 95:300–306
Altenbernd J, Wetter A, Umutlu L et al (2016) Dual-energy computed tomography for evaluation of pulmonary nodules with emphasis on metastatic lesions. Acta Radiol 57(4):437–443
Iwano S, Ito R, Umakoshi H, Ito S, Naganawa S (2015) Evaluation of lung cancer by enhanced dual-energy CT: association between three-dimensional iodine concentration and tumour differentiation. Br J Radiol 88(1055)
Rizzo S, Radice D, Femia M et al (2018) Metastatic and non-metastatic lymph nodes: quantification and different distribution of iodine uptake assessed by dual-energy CT. Eur Radiol 28(2):760–769
Große Hokamp N, Gupta A, Gilkeson RC (2019) Stratification of pulmonary nodules using quantitative iodine maps from dual-energy computed tomography. Am J Respir Crit Care Med 199(2):e3–e4
Borggrefe J, Neuhaus V-F, Le Blanc M et al (2018) Accuracy of iodine density thresholds for the separation of vertebral bone metastases from healthy-appearing trabecular bone in spectral detector computed tomography. Eur Radiol. https://doi.org/10.1007/s00330-018-5843-y
Lennartz S, Le Blanc M, Zopfs D et al (2019) Dual-energy CT-derived iodine maps: use in assessing pleural carcinomatosis. Radiology. https://doi.org/10.1148/radiol.2018181567
Uhrig M, Simons D, Ganten M-K, Hassel JC, Schlemmer H-P (2015) Histogram analysis of iodine maps from dual energy computed tomography for monitoring targeted therapy of melanoma patients. Future Oncol 11(4):591–606
Baxa J, Matouskova T, Krakorova G et al (2016) Dual-phase dual-energy CT in patients treated with erlotinib for advanced non-small cell lung cancer: possible benefits of iodine quantification in response assessment. Eur Radiol 26(8):2828–2836
Hellbach K, Sterzik A, Sommer W et al (2017) Dual energy CT allows for improved characterization of response to antiangiogenic treatment in patients with metastatic renal cell cancer. Eur Radiol 27(6):2532–2537
Bargellini I, Crocetti L, Turini FM et al (2018) Response assessment by volumetric iodine uptake measurement: preliminary experience in patients with intermediate-advanced hepatocellular carcinoma treated with Yttrium-90 radioembolization. Cardiovasc Intervent Radiol 41(9):1373–1383
Skornitzke S, Fritz F, Mayer P et al (2018) Dual-energy CT iodine maps as an alternative quantitative imaging biomarker to abdominal CT perfusion: determination of appropriate trigger delays for acquisition using bolus tracking. Br J Radiol 91(1085):20170351
Funding
This study was funded by the Else Kröner-Fresenius-Stiftung (Grant 2018_EKMS.34 to Dr. Nils Große Hokamp).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Nils Große Hokamp.
Conflict of interest
SL received research and travel support outside this specific project from Philips Healthcare. JB, DM received speaker’s honoraria from Philips Healthcare. NGH received speaker’s honoraria and travel support from Philips Healthcare. All other authors: nothing to disclose.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective
• Observational
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1650 kb)
Rights and permissions
About this article
Cite this article
Lennartz, S., Abdullayev, N., Zopfs, D. et al. Intra-individual consistency of spectral detector CT-enabled iodine quantification of the vascular and renal blood pool. Eur Radiol 29, 6581–6590 (2019). https://doi.org/10.1007/s00330-019-06266-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06266-w