Abstract
Objectives
To assess inter-platform reproducibility of ultrasonic attenuation coefficient (AC) and backscatter coefficient (BSC) estimates in adults with known/suspected nonalcoholic fatty liver disease (NAFLD).
Methods
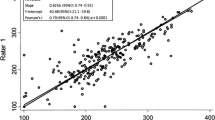
This HIPAA-compliant prospective study was approved by an institutional review board; informed consent was obtained. Participants with known/suspected NAFLD were recruited and underwent same-day liver examinations with clinical ultrasound scanner platforms from two manufacturers. Each participant was scanned by the same trained sonographer who performed multiple data acquisitions in the right liver lobe using a lateral intercostal approach. Each data acquisition recorded a B-mode image and the underlying radio frequency (RF) data. AC and BSC were calculated using the reference phantom method. Inter-platform reproducibility was evaluated for AC and log-transformed BSC (logBSC = 10log10BSC) by intraclass correlation coefficient (ICC), Pearson’s correlation, Bland-Altman analysis with computation of limits of agreement (LOAs), and within-subject coefficient of variation (wCV; applicable to AC).
Results
Sixty-four participants were enrolled. Mean AC values measured using the two platforms were 0.90 ± 0.13 and 0.94 ± 0.15 dB/cm/MHz while mean logBSC values were − 30.6 ± 5.0 and − 27.9 ± 5.6 dB, respectively. Inter-platform ICC was 0.77 for AC and 0.70 for log-transformed BSC in terms of absolute agreement. Pearson’s correlation coefficient was 0.81 for AC and 0.80 for logBSC. Ninety-five percent LOAs were − 0.21 to 0.13 dB/cm/MHz for AC, and − 9.48 to 3.98 dB for logBSC. The wCV was 7% for AC.
Conclusions
Hepatic AC and BSC are reproducible across two different ultrasound platforms in adults with known or suspected NAFLD.
Key Points
• Ultrasonic attenuation coefficient and backscatter coefficient are reproducible between two different ultrasound platforms in adults with NAFLD.
• This inter-platform reproducibility may qualify quantitative ultrasound biomarkers for generalized clinical application in patients with suspected/known NAFLD.




Similar content being viewed by others
Abbreviations
- AC:
-
Attenuation coefficient
- ANOVA:
-
Analysis of variance
- AUC:
-
Area under the receiver operating characteristic curve
- BMI:
-
Body mass index
- BSC:
-
Backscatter coefficient
- CAP:
-
Controlled attenuation parameter
- CUS:
-
Conventional ultrasonography
- FDA:
-
Food and Drug Administration
- FOI:
-
Field of interest
- HIPAA:
-
Health Insurance Portability and Accountability Act
- ICC:
-
Intraclass correlation coefficient
- logBSC:
-
Log-transformed backscatter coefficient
- LOA:
-
Limit of agreement
- MRI:
-
Magnetic resonance imaging
- NAFLD:
-
Nonalcoholic fatty liver disease
- NASH:
-
Nonalcoholic steatohepatitis
- NASH CRN:
-
Nonalcoholic Steatohepatitis Clinical Research Network
- PDFF:
-
Proton density fat fraction
- QIB:
-
Quantitative imaging biomarker
- QUS:
-
Quantitative ultrasound
- RF:
-
Radio frequency
- wCV:
-
Within-subject coefficient of variation
References
Loomba R, Sanyal AJ (2013) The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 10:686–690
Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ (2018) Mechanisms of NAFLD development and therapeutic strategies. Nat Med 24:908–922
Machado MV, Cortez-Pinto H (2013) Non-invasive diagnosis of non-alcoholic fatty liver disease. A critical appraisal. J Hepatol 58:1007–1019
Park CC, Nguyen P, Hernandez C et al (2017) Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology 152:598–607
Reeder SB, Cruite I, Hamilton G, Sirlin CB (2011) Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging 34:729–749
Artz NS, Hines CD, Brunner ST et al (2012) Quantification of hepatic steatosis with dual-energy computed tomography: comparison with tissue reference standards and quantitative magnetic resonance imaging in the ob/ob mouse. Invest Radiol 47:603–610
Andre MP, Han A, Heba E et al (2014) Accurate diagnosis of nonalcoholic fatty liver disease in human participants via quantitative ultrasound. In: 2014 IEEE International Ultrasonics Symposium. pp 2375–2377
Lin SC, Heba E, Wolfson T et al (2015) Noninvasive diagnosis of nonalcoholic fatty liver disease and quantification of liver fat using a new quantitative ultrasound technique. Clin Gastroenterol Hepatol 13:1337–1345.e6
Paige JS, Bernstein GS, Heba E et al (2017) A pilot comparative study of quantitative ultrasound, conventional ultrasonography, and magnetic resonance imaging for predicting histology-determined steatosis grade in adult nonalcoholic fatty liver disease. AJR Am J Roentgenol 208:W1–W10
Oelze ML, Mamou J (2016) Review of quantitative ultrasound: envelope statistics and backscatter coefficient imaging and contributions to diagnostic ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control 63:336–351
Sasso M, Beaugrand M, de Ledinghen V et al (2010) Controlled attenuation parameter (CAP): a novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol 36:1825–1835
Caussy C, Alquiraish MH, Nguyen P et al (2018) Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology 67:1348–1359
Imbault M, Faccinetto A, Osmanski BF et al (2017) Robust sound speed estimation for ultrasound-based hepatic steatosis assessment. Phys Med Biol 62:3582–3598
Sullivan DC, Obuchowski NA, Kessler LG et al (2015) Metrology standards for quantitative imaging biomarkers. Radiology 277:813–825
Han A, Andre MP, Erdman JW, Loomba R, Sirlin CB, O’Brien WD (2017) Repeatability and reproducibility of a clinically based QUS phantom study and methodologies. IEEE Trans Ultrason Ferroelectr Freq Control 64:218–231
Han A, Andre MP, Deiranieh L et al (2018) Repeatability and reproducibility of ultrasonic attenuation coefficient and backscatter coefficient measured in the right lobe of the liver in adults with known or suspected nonalcoholic fatty liver disease. J Ultrasound Med 37:1913–1927
Han A, Labyed Y, Sy EZ et al (2018) Inter-sonographer reproducibility of quantitative ultrasound outcomes and shear wave speed measured in the right lobe of the liver in adults with known or suspected nonalcoholic fatty liver disease. Eur Radiol 28:4992–5000
Kleiner DE, Brunt EM, Van Natta M et al (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41:1313–1321
Yao LX, Zagzebski JA, Madsen EL (1990) Backscatter coefficient measurements using a reference phantom to extract depth-dependent instrumentation factors. Ultrason Imaging 12:58–70
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Shrout PE, Fleiss JL (1979) Intraclass correlations: uses in assessing rater reliability. Psychol Bull 86:420–428
Raunig DL, McShane LM, Pennello G et al (2015) Quantitative imaging biomarkers: a review of statistical methods for technical performance assessment. Stat Methods Med Res 24:27–67
Fleiss JL (1999) The design and analysis of clinical experiments. Wiley, New York, pp 1–32
Trout AT, Serai S, Mahley AD et al (2016) Liver stiffness measurements with MR elastography: agreement and repeatability across imaging systems, field strengths, and pulse sequences. Radiology 281:793–804
Nobili V, Vizzutti F, Arena U et al (2008) Accuracy and reproducibility of transient elastography for the diagnosis of fibrosis in pediatric nonalcoholic steatohepatitis. Hepatology 48:442–448
Bota S, Sporea I, Sirli R, Popescu A, Danila M, Costachescu D (2012) Intra- and interoperator reproducibility of acoustic radiation force impulse (ARFI) elastography--preliminary results. Ultrasound Med Biol 38:1103–1108
Guerrero QW, Fan L, Brunke S, Milkowski A, Rosado-Mendez IM, Hall TJ (2018) Power spectrum consistency among systems and transducers. Ultrasound Med Biol, online. https://doi.org/10.1016/j.ultrasmedbio.2018.05.013
Ferraioli G, Filice C, Castera L et al (2015) WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 3: liver. Ultrasound Med Biol 41:1161–1179
Serai SD, Dillman JR, Trout AT (2017) Proton density fat fraction measurements at 1.5- and 3-T hepatic MR imaging: same-day agreement among readers and across two imager manufacturers. Radiology 284:244–254
Mashhood A, Railkar R, Yokoo T et al (2013) Reproducibility of hepatic fat fraction measurement by magnetic resonance imaging. J Magn Reson Imaging 37:1359–1370
Kang GH, Cruite I, Shiehmorteza M et al (2011) Reproducibility of MRI-determined proton density fat fraction across two different MR scanner platforms. J Magn Reson Imaging 34:928–934
Hernando D, Sharma SD, Aliyari Ghasabeh M et al (2017) Multi-site, multi-vendor validation of the accuracy and reproducibility of proton-density fat- fraction quantification at 1.5T and 3T using a fat-water phantom. Magn Reson Med 77:1516–1524
Acknowledgements
The authors thank the research participants for making this study possible, the sonographers, Elise Housman, Susan Lynch, and Minaxi Trivedi, for the dedicated contributions and expertise, the clinical coordinator Vivian Montes for her outstanding organization of the many moving parts, and the pathologist Mark A. Valasek, MD, PhD, for reading the histology and determining the steatosis grade and fibrosis stage.
Funding
This study has received funding by the National Institutes of Health (R01DK106419), Siemens Healthineers USA, and GE Healthcare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Claude B. Sirlin, MD (University of California, San Diego).
Conflict of interest
The authors of this manuscript declare relationships with the following companies:
The work is supported in part by research grants from Siemens Healthineers USA and GE Healthcare. The use of the Siemens S3000 scanner was loaned to the University of California, San Diego under a research agreement with Siemens Healthineers, USA. The use of the GE Logiq E9 scanner was loaned to the University of California, San Diego under a research agreement with GE Healthcare.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in [17].
Methodology
• prospective
• cross-sectional study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Han, A., Zhang, Y.N., Boehringer, A.S. et al. Inter-platform reproducibility of ultrasonic attenuation and backscatter coefficients in assessing NAFLD. Eur Radiol 29, 4699–4708 (2019). https://doi.org/10.1007/s00330-019-06035-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06035-9