Abstract
Objectives
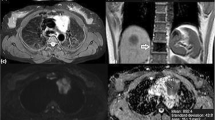
To prospectively evaluate usefulness of the apparent diffusion coefficient (ADC) in differentiating anterior mediastinal lymphoma from nonsuppressing normal thymus on chemical-shift MR, and to look at the relationship between patient age and ADC.
Methods
Seventy-three young subjects (25 men, 48 women; age range, 9-29 years), who underwent chemical-shift MR and diffusion-weighted MR were divided into a normal thymus group (group A, 40 subjects), and a lymphoma group (group B, 33 patients). For group A, all subjects had normal thymus with no suppression on opposed-phase chemical-shift MR. Two readers measured the signal intensity index (SII) and ADC. Differences in SII and ADC between groups were tested using t-test. ADC was correlated with age using Pearson correlation coefficient.
Results
Mean SII±standard deviation was 2.7±1.8% for group A and 2.2±2.4% for group B, with no significant difference between groups (P=.270). Mean ADC was 2.48±0.38x10-3mm2/s for group A and 1.24±0.23x10-3mm2/s for group B. A significant difference between groups was found (P<.001), with no overlap in range. Lastly, significant correlation was found between age and ADC (r=0.935, P<.001) in group A.
Conclusions
ADC of diffusion-weighted MR is a noninvasive and accurate parameter for differentiating lymphoma from nonsuppressing thymus on chemical-shift MR in young subjects.
Key Points
• SII cannot differentiate mediastinal lymphoma from nonsuppressing normal thymus at visual assessment
• ADC is useful for distinguishing nonsuppressing normal thymus from mediastinal lymphoma
• ADC is more accurate than transverse-diameter and surface-area in this discrimination
• ADC of normal thymus is age dependent and increases with increasing age






Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUROC:
-
Area under the receiving operating characteristic curve
- CT:
-
Computed tomography
- MR:
-
Magnetic resonance
- PET:
-
Positron emission tomography
- ROI:
-
Region of interest
- SII:
-
Signal intensity index
References
MC MI, Flores EJ, Shepard JA, Ackman JB (2016) Pitfalls in the imaging and interpretation of benign thymic lesions: how thymic MRI can help. AJR Am J Roentgenol 206:W1–W8
Priola AM, Gned D, Veltri A, Priola SM (2016a) Chemical shift and diffusion-weighted magnetic resonance imaging of the anterior mediastinum in oncology: current clinical applications in qualitative and quantitative assessment. Crit Rev Oncol Hematol 98:335–357
Nishino M, Ashiku SK, Kocher ON, Thurer RL, Boiselle PM, Hatabu H (2006) The thymus: a comprehensive review. Radiographics 26:335–348
Priola AM, Priola SM, Ciccone G et al (2015) Differentiation of rebound and lymphoid thymic hyperplasia from anterior mediastinal tumors with dual-echo chemical-shift magnetic resonance imaging in adulthood: reliability of the chemical-shift ratio and signal-intensity index. Radiology 274:238–249
Inaoka T, Takahashi K, Iwata K et al (2005) Evaluation of normal fatty replacement of the thymus with chemical-shift MR imaging for identification of the normal thymus. J Magn Reson Imaging 22:341–346
Ackman JB, Mino-Kenudson M, Morse CR (2012) Nonsuppressing normal thymus on chemical shift magnetic resonance imaging in a young woman. J Thorac Imaging 27:196–198
Priola AM, Galetto G, Priola SM (2014) Diagnostic and functional imaging of thymic and mediastinal involvement in lymphoproliferative disorders. Clin Imaging 38:771–784
Ackman JB, Kovacina B, Carter BW et al (2013) Sex difference in normal thymic appearance in adults 20-30 years of age. Radiology 268:245–253
Blazic IM, Lilic GB, Gajic MM (2017) Quantitative assessment of rectal cancer response to neoadjuvant combined chemotherapy and radiation therapy: comparison of three methods of positioning region of interest for ADC measurements at diffusion-weighted MR imaging. Radiology 282:418–428
DMJ L, Beets GL, Mass M et al (2011) Tumor ADC measurements in rectal cancer: effect of ROI methods on ADC values and interobserver variability. Eur Radiol 21:2567–2574
Fischer MA, Nanz D, Hany T et al (2011) Diagnostic accuracy of whole-body MRI/DWI image fusion for detection of malignant tumours: a comparison with PET/CT. Eur Radiol 21:246–255
Filli L, Wurnig MC, Luechinger R, Eberhardt C, Guggenberger R, Boss A (2015) Whole-body intravoxel incoherent motion imaging. Eur Radiol 25:2049–2058
Clauser P, Marcon M, Maieron M, Zuiani C, Bazzocchi M, Baltzer PA (2016) Is there a systematic bias of apparent diffusion coefficient (ADC) measurements of the breast if measured on different workstations? An inter- and intra-reader agreement study. Eur Radiol 26:2291–2296
Priola AM, Priola SM, Parlatano D et al (2017a) Apparent diffusion coefficient measurements in diffusion-weighted magnetic resonance imaging of the anterior mediastinum: inter-observer reproducibility of five different methods of region-of-interest positioning. Eur Radiol 27:1386–1394
Ackman JB (2014) A practical guide to nonvascular thoracic magnetic resonance imaging. J Thorac Imaging 29:17–29
Abdel Razek AA, Elmorsy A, Elshafey M, Elhadedy T, Hamza O (2009) Assessment of mediastinal tumors with diffusion weighted single shot echo planar MR imaging. J Magn Reson Imaging 30:535–540
Abdel Razek AA, Elkammary S, Elmorsy AS, Elshafey M, Elhadedy T (2011) Characterization of mediastinal lymphadenopathy with diffusion-weighted imaging. Magn Reson Imaging 29:167–172
Gümüștaș S, Inan N, Sarisoy HT et al (2011) Malignant versus benign mediastinal lesions: quantitative assessment with diffusion weighted MR imaging. Eur Radiol 21:2255–2260
Gümüștaș S, Inan N, Akansel G, Bașyiğit I, Ciftçi E (2013) Differentiation of lymphoma versus sarcoidosis in the setting of mediastinal-hilar lymphadenopathy: assessment with diffusion-weighted MR imaging. Sarcoidosis Vasc Diffuse Lung Dis 30:52–59
Shin KE, Yi CA, Kim TS et al (2014) Diffusion-weighted MRI for distinguishing non-neoplastic cysts from solid masses in the mediastinum: problem-solving in mediastinal masses of indeterminate internal characteristics on CT. Eur Radiol 24:677–684
Abdel Razek AA, Khairy M, Nada N (2014) Diffusion-weighted MR imaging in thymic epithelial tumors: correlation with World Health Organization classification and clinical staging. Radiology 273:268–275
Priola AM, Priola SM, Giraudo MT et al (2016b) Diffusion-weighted magnetic resonance imaging of thymoma: ability of the apparent diffusion coefficient in predicting the World Health Organization (WHO) classification and the Masaoka-Koga staging system and its prognostic significance on disease-free survival. Eur Radiol 26:2126–2138
Coolen J, De Keyzer F, Nafteux P et al (2015) Malignant pleural mesothelioma: visual assessment by using pleural pointillism at diffusion-weighted MR imaging. Radiology 274:576–584
Priola AM, Priola SM, Gned D et al (2017b) Diffusion-weighted quantitative MRI of pleural abnormalities: intra- and interobserver variability in the apparent coefficient measurements. J Magn Reson Imaging 46:769–782
Bossuyt PM, Reitsma JB, Bruns DE et al (2015) STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. Radiology 277:826–832
Araki T, Nishino M, Gao W et al (2016) Normal thymus in adults: appearance on CT and associations with age, sex, BMI and smoking. Eur Radiol 26:15–24
Priola AM, Priola SM, Gned D, Giraudo MT, Fornari A, Veltri A (2016c) Comparison of CT and chemical-shift MRI for differentiating thymoma from non-thymomatous conditions in myasthenia gravis: value of qualitative and quantitative assessment. Clin Radiol 71:e157–e169
Priola AM, Priola SM, Gned D et al (2016d) Diffusion-weighted magnetic resonance quantitative imaging to diagnose benign conditions from malignancies of the anterior mediastinum: improvement of diagnostic accuracy by comparing perfusion-free to perfusion-sensitive measurements of the apparent diffusion coefficient. J Magn Reson Imaging 44:758–769
Padhani AR, Liu G, Koh DM et al (2009) Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 11:102–125
Coolen J, Vansteenkiste J, De Keyzer F et al (2014) Characterisation of solitary pulmonary lesions combining visual perfusion and quantitative diffusion MR imaging. Eur Radiol 24:531–541
Nougaret S, Vargas HA, Lakhman Y et al (2016) Intravoxel incoherent motion-derived histogram metrics for assessment of response after combined chemotherapy and radiation therapy in rectal cancer: initial experience and comparison between single-section and volumetric analyses. Radiology 280:446–454
Priola AM, Veltri A, Priola SM (2016e) Importance of different region-of-interest protocols for the apparent diffusion coefficient measurement of tumors in diffusion-weighted magnetic resonance imaging. J Magn Reson Imaging 44:1056
Ahlawat S, Khandheria P, Del Grande F et al (2016) Interobserver variability of selective region-of-interest measurement protocols for quantitative diffusion weighted imaging in soft tissue masses: comparison with whole tumor volume measurements. J Magn Reson Imaging 43:446–454
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Littooij AS, Kwee TC, Barber I et al (2014) Whole-body MRI for initial staging of paediatric lymphoma: prospective comparison to an FDG-PET/CT-based reference standard. Eur Radiol 24:1153–1165
Punwani S, Taylor SA, Saad ZZ et al (2013) Diffusion-weighted MRI of lymphoma: prognostic utility and implications for PET/MRI? Eur J Nucl Med Mol Imaging 40:373–385
Ackman JB, Gaissert HA, Lanuti M et al (2016) Impact of nonvascular thoracic MR imaging on the clinical decision making of thoracic surgeons: a 2-year prospective study. Radiology 280:464–474
Adam SZ, Nikolaidis P, Horowitz JM et al (2016) Chemical shift MR of the adrenal gland: principles, pitfalls, and applications. Radiographics 36:414–432
Priola AM, Priola SM (2014) Imaging of thymus in myasthenia gravis: from thymic hyperplasia to thymic tumor. Clin Radiol 69:e230–e245
Inaoka T, Takahashi K, Mineta M et al (2007) Thymic hyperplasia and thymus gland tumors: differentiation with chemical shift MR imaging. Radiology 243:869–876
Priola AM, Priola SM (2009) Primary mediastinal Hodgkin lymphoma and rebound thymic hyperplasia: differentiation with chemical-shift magnetic resonance imaging after treatment. Int J Hematol 90:8–10
Gawande RS, Khurana A, Messing S et al (2012) Differentiation of normal thymus from anterior mediastinal lymphoma and lymphoma recurrence at pediatric PET/CT. Radiology 262:613–622
Jerushalmi J, Frenkel A, Bar-Shalom R, Khoury J, Israel O (2009) Physiologic thymic uptake of 18F-FDG in children and young adults: a PET-CT evaluation of incidence, patterns, and relationship to treatment. J Nucl Med 50:849–853
Yabuuchi H, Matsuo Y, Abe K et al (2015) Anterior mediastinaal solid tumours in adults: characterization using dynamic contrast-enhanced MRI, diffusion-weighted MRI, and FDG-PET/CT. Clin Radiol 70:1289–1298
Seki S, Koyama H, Ohno Y et al (2014) Diffusion-weighted MR imaging vs. multi-detector row-CT: direct comparison of capability for assessment of management needs for anterior mediastinal solitary tumors. Eur J Radiol 83:835–842
Park BK, Kim CK, Kim B, Lee JH (2007) Comparison of delayed enhanced CT and chemical shift MR for evaluating hyperattenuating incidental adrenal masses. Radiology 243:760–765
Liu D, Kitajima M, Awai K et al (2006) Ectopic cervical thymus in an infant. Radiat Med 24:452–455
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Adriano M. Priola.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, with products or services that may be related to the subject matter of the article.
Statistics and biometry
One of the authors (Maria T. Giraudo) has significant statistical expertise.
Ethical approval
Institutional Review Board approval was obtained.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Methodology
1. prospective
2. observational
3. performed at one institution
Rights and permissions
About this article
Cite this article
Priola, A.M., Priola, S.M., Gned, D. et al. Nonsuppressing normal thymus on chemical-shift MR imaging and anterior mediastinal lymphoma: differentiation with diffusion-weighted MR imaging by using the apparent diffusion coefficient. Eur Radiol 28, 1427–1437 (2018). https://doi.org/10.1007/s00330-017-5142-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-5142-z