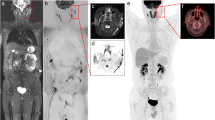
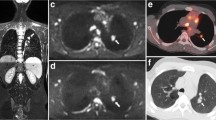
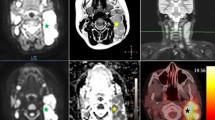
Abstract
Objectives
We investigated the correlation between the apparent diffusion coefficient (ADC) and Ki-67 index using diffusion-weighted whole-body imaging with background body signal suppression (DWIBS), and their utility in evaluating malignant lymphoma cell proliferation.
Materials and Methods
Seventy-four patients with malignant lymphoma underwent DWIBS within 1 week before pathological confirmation. The ADC value was measured at the site of the pathological examination, and specimens were also stained with Ki-67. The ADC values and Ki-67 indices in aggressive non-Hodgkin's lymphoma (NHL), indolent NHL, and Hodgkin's lymphoma (HL) were compared using Spearman's rank correlation coefficient and the Kruskal-Wallis test.
Results
The Ki-67 indices and ADC values were inversely correlated (r = -0.289, p = 0.0125); the differences in the Ki-67 index between aggressive NHL, indolent NHL, and HL were significant (p < 0.001); this was confirmed by the Nemenyi test except for indolent NHL vs. HL. The ADC values were significantly different between the types of lymphoma (p = 0.013); the Nemenyi test showed a significant difference only between aggressive NHL and HL.
Conclusions
The Ki-67 indices and ADC values are inversely correlated in patients with lymphoma, combining DWIBS and ADC values can evaluate the proliferation level of malignant lymphoma cells noninvasively.
Key points
• By using DWIBS, malignant lymphoma cell proliferation can be assessed noninvasively.
• The ADC value and Ki-67 index are significantly and inversely correlated.
• The ADC values were lower in aggressive NHL than in HL.
• The ADC values of aggressive and indolent NHL were not significantly different.





Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- DWIBS:
-
Diffusion-weighted whole-body imaging with background body signal suppression
- NHL:
-
Non-Hodgkin's lymphoma
- HL:
-
Hodgkin's lymphoma
- MRI:
-
Magnetic resonance imaging
- TI:
-
Inversion time
- DWI:
-
Diffusion-weighted imaging
- TR:
-
Repetition time
- TE:
-
Echo time
- FOV:
-
Field of view
- ROI:
-
Region of interest
- NK:
-
Natural killer
- MCL:
-
Mantle cell lymphoma
- IPI:
-
International Prognostic Index
References
Zhang J, Grubor V, Love CL et al (2013) Genetic heterogeneity of diffuse large B-cell lymphoma. Proc Natl Acad Sci U S A 110:1398–1403
Abdulqadhr G, Molin D, Aström G et al (2011) Whole-body diffusion-weighted imaging compared with FDG-PET/CT in staging of lymphoma patients. Acta Radiol 52:173–180
Gu J, Chan T, Zhang J et al (2011) Whole-body diffusion-weighted imaging: the added value to whole-body MRI at initial diagnosis of lymphoma. AJR Am J Roentgenol 197:W384–W391
Chen L, Liu M, Bao J et al (2013) The correlation between apparent diffusion coefficient and tumor cellularity in patients: a meta-analysis. PLoS One 8:e79008
Jeon JY, Chung HW, Lee MH et al (2016) Usefulness of diffusion-weighted MR imaging for differentiating between benign and malignant superficial soft tissue tumours and tumour-like lesions. Br J Radiol 89:20150929
Horger M, Claussen C, Kramer U et al (2014) Very early indicators of response to systemic therapy in lymphoma patients based on alterations in water diffusivity--a preliminary experience in 20 patients undergoing whole-body diffusion-weighted imaging. Eur J Radiol 83:1655–1664
Barajas RF Jr, Rubenstein JL, Chang JS et al (2010) Diffusion-weighted MR imaging derived apparent diffusion coefficient is predictive of clinical outcome in primary central nervous system lymphoma. AJNR Am J Neuroradiol 31:60–66
Valles FE, Perez-Valles CL, Regalado S et al (2013) Combined diffusion and perfusion MR imaging as biomarkers of prognosis in immunocompetent patients with primary central nervous system lymphoma. AJNR Am J Neuroradiol 34:35–40
Schob S, Meyer J, Gawlitza M et al (2016) Diffusion-Weighted MRI Reflects Proliferative Activity in Primary CNS Lymphoma. PLoS One 11:e0161386
Broyde A, Boycov O, Strenov Y et al (2009) Role and prognostic significance of the Ki-67 index in non-Hodgkin's lymphoma. Am J Hematol 84:338–343
Cuenca X, Xhaard A, Mounier N (2009) Prognostic factors in Hodgkin and non-Hodgkin lymphomas. Bull Cancer 96:461–473
Swerdlow SH, Campo E, Harris NL et al (2008) WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press, Lyon, France
Huang HH, Xiao F, Chen FY et al (2012) Reassessment of the prognostic value of the International Prognostic Index and the revised InternationalPrognostic Index in patients with diffuse large B-cell lymphoma: A multicentre study. Exp Ther Med 4:475–480
Sehn LH, Berry B, Chhanabhai M et al (2007) The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 109:1857–1861
Prochazka KT, Melchardt T, Posch F et al (2016) NCCN-IPI score-independent prognostic potential of pretreatment uric acid levels for clinical outcome of diffuse large B-cell lymphoma patients. Br J Cancer 115:1264–1272
Gerdes J, Schwab U, Lemke H et al (1983) Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer 31:13–20
Jakovic LR, Mihaljevic BS, Jovanovic MD et al (2007) The expression of Ki-67 and Bcl-2 in Hodgkin's lymphoma: correlation with the International Prognostic Score and bulky disease: a study by the Serbian Lymphoma Study Group (SLG). Med Oncol 24:45–53
Hoster E, Rosenwald A, Berger F et al (2016) Prognostic value of Ki-67 index, cytology, and growth pattern in mantle-cell lymphoma: results from randomized trials of the European Mantle Cell Lymphoma Network. J Clin Oncol 34:1386–1394
Küppers R (2009) The biology of Hodgkin's lymphoma. Nat Rev Cancer 9:15–27
Huang Z, Xu X, Meng X et al (2015) Correlations between ADC values and molecular markers of Ki-67 and HIF-1α in hepatocellular carcinoma. Eur J Radiol 84:2464–2469
Meng X, Li H, Kong L et al (2016) MRI In rectal cancer: Correlations between MRI features and molecular markers Ki-67, HIF-1α, and VEGF. J Magn Reson Imaging 44:594–600
Guo AC, Cummings TJ, Dash RC et al (2002) Lymphomas and high-grade astrocytomas: comparison of water diffusibility and histologic characteristics. Radiology 224:177–183
Wu X, Pertovaara H, Dastidar P et al (2013) ADC measurements in diffuse large B-cell lymphoma and follicular lymphoma: a DWI and cellularity study. Eur J Radiol 82:e158–e164
Fernebro J, Engellau J, Persson A et al (2007) Standardizing evaluation of sarcoma proliferation- higher Ki-67 expression in the tumor periphery than the center. APMIS 115:707–712
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Jingliang Cheng.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Funding
This study has received funding by a key project grant from the Technology Department of Henan Province, China. No.112102310703
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic study
• performed at one institution
Rights and permissions
About this article
Cite this article
Sun, M., Cheng, J., Zhang, Y. et al. Application of DWIBS in malignant lymphoma: correlation between ADC values and Ki-67 index. Eur Radiol 28, 1701–1708 (2018). https://doi.org/10.1007/s00330-017-5135-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-5135-y