Abstract
Objectives
Detection of subclinical hepatic encephalopathy in children is difficult. We aimed to assess the changes in imaging of the central nervous system in children with chronic liver disease using MR imaging, diffusion, and 1H -spectroscopy.
Methods
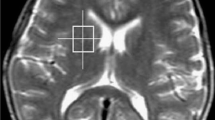
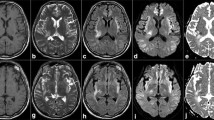
Forty three children with chronic liver disease and/or porto-systemic shunting (111.4±56.9 months) and 24 controls (72.0±51.8 months) underwent brain MRI/spectroscopy on a 1.5T to examine T1, T2, ADC, Cho/Cr, ml/Cr, Glx/Cr ratio spectroscopy in the globus pallidus. Patients were divided into 3 groups according to the ratios of globus pallidus/putamen T1 signal : isointense (i), hyperintense (h), much more hyperintense (h+). The relationship with clinical and biological data was analyzed.
Results
T1 signal intensity and ml/Cr were significantly different between controls and group h+ (p=0.001). ADC did not differ significantly between groups. Age correlated strongly with the presence of a T1 signal ratio (p > 0.001). There was no correlation between imaging findings and biological parameters.
Conclusions
In children with chronic liver disease and/or porto-systemic shunting, the presence of a hyperintense T1 signal in the globus pallidus correlated strongly with age. Biological and clinical parameters were not predictive of these changes. MRI may become a useful screening tool for hepatic encephalopathy in children.
Key Points
• Children with chronic liver disease should undergo brain MRI during their follow-up
• T1 hyperintensity of globus pallidus is suggestive of liver-related CNS involvement
• MRS mI/Cr is decreased in children with chronic liver disease
• Biological parameters (ammonium) were not predictive of hepatic encephalopathy
• Duration of chronic liver disease may be causative the hepatic encephalopathy


Similar content being viewed by others
Abbreviations
- CLD:
-
Chronic liver disease
- mHE:
-
Minimal hepatic encephalopathy
- HE:
-
Hepatic encephalopathy
- PSS:
-
Porto-systemic shunting
- GP:
-
Globus pallidus
- ROI:
-
Region-of-interest
- mI:
-
myoInositol
- Gln:
-
Glutamine
- Cho:
-
Choline
References
Grover VP, Tognarelli JM, Massie N, Crossey MM, Cook NA, Taylor-Robinson SD (2015) The why and wherefore of hepatic encephalopathy. Int J Gen Med 8:381–390
Watanabe A (1998) Cerebral changes in hepatic encephalopathy. J Gastroenterol Hepatol 13:752–760
Dharel N, Bajaj JS (2015) Definition and nomenclature of hepatic encephalopathy. J Clin Exp Hepatol 5:S37–S41
Butterworth RF (2016) BR (2015) Pathogenesis of hepatic encephalopathy in cirrhosis: the concept of synergism revisited. Metab Brain Dis 31:1211–1215
Spahr L, Butterworth RF, Fontaine S et al (1996) Increased blood manganese in cirrhotic patients: relationship to pallidal magnetic resonance signal hyperintensity and neurological symptoms. Hepatology 24:1116–1120
Pomier-Layrargues G, Spahr L, Butterworth RF (1995) Increased manganese concentrations in pallidum of cirrhotic patients. Lancet 345:735
Krieger D, Krieger S, Jansen O, Gass P, Theilmann L, Lichtnecker H (1995) Manganese and chronic hepatic encephalopathy. Lancet 346:270–274
Rose C, Butterworth RF, Zayed J et al (1999) Manganese deposition in basal ganglia structures results from both portal-systemic shunting and liver dysfunction. Gastroenterology 117:640–644
Poveda MJ, Bernabeu A, Concepcion L et al (2010) Brain edema dynamics in patients with overt hepatic encephalopathy A magnetic resonance imaging study. Neuroimage 52:481–487
Sorensen LG, Neighbors K, Martz K et al (2014) Longitudinal study of cognitive and academic outcomes after pediatric liver transplantation. J Pediatr 165:e62
Gupta RK, Dhiman RK (2003) Magnetic resonance imaging and spectroscopy in hepatic encephalopathy. Indian J Gastroenterol 22:S45–S49
McPhail MJ, Taylor-Robinson SD (2010) The role of magnetic resonance imaging and spectroscopy in hepatic encephalopathy. Metab Brain Dis 25:65–72
Goel A, Yadav S, Saraswat V et al (2010) Cerebral oedema in minimal hepatic encephalopathy due to extrahepatic portal venous obstruction. Liver Int 30:1143–1151
Dineen RA, Sibtain N, Karani JB, Lenthall RK (2008) Cerebral manifestations in liver disease and transplantation. Clin Radiol 63:586–599
Rovira A, Alonso J, Cordoba J (2008) MR imaging findings in hepatic encephalopathy. AJNR Am J Neuroradiol 29:1612–1621
Razek AA, Abdalla A, Ezzat A, Megahed A, Barakat T (2014) Minimal hepatic encephalopathy in children with liver cirrhosis: diffusion-weighted MR imaging and proton MR spectroscopy of the brain. Neuroradiology 56:885–891
Yadav SK, Srivastava A, Srivastava A et al (2010) Encephalopathy assessment in children with extra-hepatic portal vein obstruction with MR, psychometry and critical flicker frequency. J Hepatol 52:348–354
Srivastava A, Yadav SK, Lal R et al (2010) Effect of surgical portosystemic shunt on prevalence of minimal hepatic encephalopathy in children with extrahepatic portal venous obstruction: assessment by magnetic resonance imaging and psychometry. J Pediatr Gastroenterol Nutr 51:766–772
Sijens PE, Alkefaji H, Lunsing RJ et al (2008) Quantitative multivoxel 1H MR spectroscopy of the brain in children with acute liver failure. Eur Radiol 18:2601–2609
Foerster BR, Conklin LS, Petrou M, Barker PB, Schwarz KB (2009) Minimal hepatic encephalopathy in children: evaluation with proton MR spectroscopy. AJNR Am J Neuroradiol 30:1610–1613
Stewart SM, Campbell RA, McCallon D, Waller DA, Andrews WS (1992) Cognitive patterns in school-age children with end-stage liver disease. J Dev Behav Pediatr 13:331–338
Caudle SE, Katzenstein JM, Karpen SJ, McLin VA (2010) Language and motor skills are impaired in infants with biliary atresia before transplantation. J Pediatr 156:936–940, 940 e931
Morgan MY, Amodio P, Cook NA et al (2015) Qualifying and quantifying minimal hepatic encephalopathy. Metab Brain Dis. doi:10.1007/s11011-015-9726-5
Lauridsen MM, Poulsen L, Rasmussen CK et al (2015) Effects of common chronic medical conditions on psychometric tests used to diagnose minimal hepatic encephalopathy. Metab Brain Dis. doi:10.1007/s11011-015-9741-6
Dommergues JP, Letierce A, Gravereau L, Plainguet F, Bernard O, Debray D (2010) Current lifestyle of young adults after liver transplantation during childhood. Am J Transplant 10:1634–1642
D'Antiga L, Betalli P, De Angelis P et al (2015) Interobserver agreement on endoscopic classification of oesophageal varices in children. J Pediatr Gastroenterol Nutr 61:176–181
Gana JC, Turner D, Mieli-Vergani G et al (2011) A clinical prediction rule and platelet count predict esophageal varices in children. Gastroenterology 141:2009–2016
Ochi T, Taoka T, Akashi T et al (2011) Discrepancy in T1 and T2 shortening of the globus pallidus in hepatic insufficiency: evaluation by susceptibility-weighted imaging. Magn Reson Med Sci 10:79–83
D'Antiga L, Dacchille P, Boniver C et al (2014) Clues for minimal hepatic encephalopathy in children with noncirrhotic portal hypertension. J Pediatr Gastroenterol Nutr 59:689–694
Caudle SE, Katzenstein JM, Karpen S, McLin V (2012) Developmental assessment of infants with biliary atresia: differences between boys and girls. J Pediatr Gastroenterol Nutr 55:384–389
Kreis R, Ross BD, Farrow NA, Ackerman Z (1992) Metabolic disorders of the brain in chronic hepatic encephalopathy detected with H-1 MR spectroscopy. Radiology 182:19–27
Lee JH, Seo DW, Lee YS et al (1999) Proton magnetic resonance spectroscopy (1H-MRS) findings for the brain in patients with liver cirrhosis reflect the hepatic functional reserve. Am J Gastroenterol 94:2206–2213
Sugimoto R, Iwasa M, Maeda M et al (2008) Value of the apparent diffusion coefficient for quantification of low-grade hepatic encephalopathy. Am J Gastroenterol 103:1413–1420
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Sylviane Hanquinet.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Funding
The authors state that this work has not received any funding.
Statistics and biometry
One of the authors has significant statistical expertise.
Ethical approval
Institutional Review Board approval was obtained.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Rights and permissions
About this article
Cite this article
Hanquinet, S., Morice, C., Courvoisier, D.S. et al. Globus pallidus MR signal abnormalities in children with chronic liver disease and/or porto-systemic shunting. Eur Radiol 27, 4064–4071 (2017). https://doi.org/10.1007/s00330-017-4808-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-4808-x