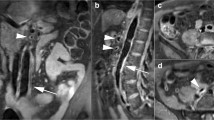
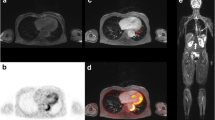
Abstract
Objectives
To evaluate the feasibility of T1w-3D black-blood turbo spin echo (TSE) sequence with variable flip angles for the diagnosis of thoracic large vessel vasculitis (LVV).
Methods
Thirty-five patients with LVV, diagnosed according to the current standard of reference, and 35 controls were imaged at 3.0T using 1.2 × 1.3 × 2.0 mm3 fat-suppressed, T1w-3D, modified Volumetric Isotropic TSE Acquisition (mVISTA) pre- and post-contrast. Applying a navigator and peripheral pulse unit triggering (PPU), the total scan time was 10–12 min. Thoracic aorta and subclavian and pulmonary arteries were evaluated for image quality (IQ), flow artefact intensity, diagnostic confidence, concentric wall thickening and contrast enhancement (CWT, CCE) using a 4-point scale.
Results
IQ was good in all examinations (3.25 ± 0.72) and good to excellent in 342 of 408 evaluated segments (83.8 %), while 84.1 % showed no or minor flow artefacts. The interobserver reproducibility for the identification of CCE and CWT was 0.969 and 0.971 (p < 0.001) with an average diagnostic confidence of 3.47 ± 0.64. CCE and CWT were strongly correlated (Cohen’s k = 0.87; P < 0.001) and significantly more frequent in the LVV-group (52.8 % vs. 1.0 %; 59.8 % vs. 2.4 %; P < 0.001).
Conclusions
Navigated fat-suppressed T1w-3D black-blood MRI with PPU-triggering allows diagnosis of thoracic LVV.
Key Points
• Cross-sectional imaging is frequently applied in the diagnosis of LVV.
• Navigated, PPU-triggered, T1w-3D mVISTA pre- and post contrast takes 10–12 min.
• In this prospective, single-centre study, T1w-3D mVISTA accurately depicted large thoracic vessels.
• T1w-3D mVISTA visualized CWT/CCW as correlates of mural inflammation in LVV.
• T1w-3D mVISTA might be an alternative diagnostic tool without ionizing radiation.



Similar content being viewed by others
Abbreviations
- 2D:
-
Two-dimensional
- 3D:
-
Three-dimensional
- ACR:
-
American College of Rheumatology
- BB:
-
Black blood
- CDUS:
-
Colour duplex ultrasound
- CCE:
-
Concentric contrast enhancement
- CWT:
-
Concentric wall thickening
- DCL:
-
Diagnostic confidence level
- ETL:
-
Echo train length
- FAI:
-
Flow artefact intensity
- FDG:
-
[18F]fluorodeoxyglucose
- LVV:
-
Large vessel vasculitis
- MRI:
-
Magnetic resonance imaging
- PET/CT:
-
Positron emission tomography/computed tomography
- T1w:
-
T1-weighted
- TAB:
-
Temporal artery biopsy
- TSE:
-
Turbo spin-echo
- mVISTA:
-
Modified Volumetric ISotropic TSE Acquisition
References
Arend WP, Michel BA, Bloch DA et al (1990) The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 33:1129–1134
Hunder GG, Bloch DA, Michel BA et al (1990) The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 33:1122–1128
Monach PA (2014) Biomarkers in vasculitis. Curr Opin Rheumatol 26:24–30
Fries JF, Hunder GG, Bloch DA et al (1990) The American College of Rheumatology 1990 criteria for the classification of vasculitis. Summary. Arthritis Rheum 33:1135–1136
Nesher G (2014) The diagnosis and classification of giant cell arteritis. J Autoimmun 48–49:73–75
de Souza AW, de Carvalho JF (2014) Diagnostic and classification criteria of Takayasu arteritis. J Autoimmun 48–49:79–83
Mukhtyar C, Guillevin L, Cid MC et al (2009) EULAR recommendations for the management of primary small and medium vessel vasculitis. Ann Rheum Dis 68:310–317
Grayson PC, Maksimowicz-McKinnon K, Clark TM et al (2012) Distribution of arterial lesions in Takayasu’s arteritis and giant cell arteritis. Ann Rheum Dis 71:1329–1334
Park MC, Lee SW, Park YB, Chung NS, Lee SK (2005) Clinical characteristics and outcomes of Takayasu’s arteritis: analysis of 108 patients using standardized criteria for diagnosis, activity assessment, and angiographic classification. Scand J Rheumatol 34:284–292
Ohigashi H, Haraguchi G, Konishi M et al (2012) Improved prognosis of Takayasu arteritis over the past decade--comprehensive analysis of 106 patients. Circ J 76:1004–1011
Evans JM, O'Fallon WM, Hunder GG (1995) Increased incidence of aortic aneurysm and dissection in giant cell (temporal) arteritis. A population-based study. Ann Intern Med 122:502–507
Nuenninghoff DM, Hunder GG, Christianson TJ, McClelland RL, Matteson EL (2003) Incidence and predictors of large-artery complication (aortic aneurysm, aortic dissection, and/or large-artery stenosis) in patients with giant cell arteritis: a population-based study over 50 years. Arthritis Rheum 48:3522–3531
Gonzalez-Gay MA, Garcia-Porrua C, Pineiro A, Pego-Reigosa R, Llorca J, Hunder GG (2004) Aortic aneurysm and dissection in patients with biopsy-proven giant cell arteritis from northwestern Spain: a population-based study. Medicine (Baltimore) 83:335–341
Cyran CC, Sourbron S, Bochmann K et al (2011) Quantification of supra-aortic arterial wall inflammation in patients with arteritis using high resolution dynamic contrast-enhanced magnetic resonance imaging: initial results in correlation to [18F]-FDG PET/CT. Investig Radiol 46:594–599
Muto G, Yamashita H, Takahashi Y et al (2014) Large vessel vasculitis in elderly patients: early diagnosis and steroid-response evaluation with FDG-PET/CT and contrast-enhanced CT. Rheumatol Int. doi:10.1007/s00296-014-2985-3
Bartels AL, Zeebregts CJ, Bijl M, Tio RA, Slart RH (2009) Fused FDG-PET and MRI imaging of Takayasu arteritis in vertebral arteries. Ann Nucl Med 23:753–756
Bley TA, Wieben O, Uhl M et al (2005) Integrated head-thoracic vascular MRI at 3 T: assessment of cranial, cervical and thoracic involvement of giant cell arteritis. MAGMA 18:193–200
Pfefferkorn T, Schuller U, Cyran C et al (2010) Giant cell arteritis of the Basal cerebral arteries: correlation of MRI, dsa, and histopathology. Neurology 74:1651–1653
Saam T, Habs M, Pollatos O et al (2010) High-resolution black-blood contrast-enhanced T1 weighted images for the diagnosis and follow-up of intracranial arteritis. Br J Radiol 83:e182–e184
Mani V, Itskovich VV, Szimtenings M et al (2004) Rapid extended coverage simultaneous multisection black-blood vessel wall MR imaging. Radiology 232:281–288
Bley TA, Wieben O, Uhl M, Thiel J, Schmidt D, Langer M (2005) High-resolution MRI in giant cell arteritis: imaging of the wall of the superficial temporal artery. AJR Am J Roentgenol 184:283–287
Busse RF, Hariharan H, Vu A, Brittain JH (2006) Fast spin echo sequences with very long echo trains: design of variable refocusing flip angle schedules and generation of clinical T2 contrast. Magn Reson Med 55:1030–1037
Busse RF, Brau AC, Vu A et al (2008) Effects of refocusing flip angle modulation and view ordering in 3D fast spin echo. Magn Reson Med 60:640–649
Mugler JP 3rd (2014) Optimized three-dimensional fast-spin-echo MRI. J Magn Reson Imaging 39:745–767
Fan Z, Zhang Z, Chung YC et al (2010) Carotid arterial wall MRI at 3T using 3D variable-flip-angle turbo spin-echo (TSE) with flow-sensitive dephasing (FSD). J Magn Reson Imaging 31:645–654
Sakurai K, Miura T, Sagisaka T et al (2013) Evaluation of luminal and vessel wall abnormalities in subacute and other stages of intracranial vertebrobasilar artery dissections using the volume isotropic turbo-spin-echo acquisition (VISTA) sequence: a preliminary study. J Neuroradiol 40:19–28
Qiao Y, Steinman DA, Qin Q et al (2011) Intracranial arterial wall imaging using three-dimensional high isotropic resolution black blood MRI at 3.0 Tesla. J Magn Reson Imaging 34:22–30
Treitl KM, Treitl M, Kooijman-Kurfuerst H et al (2015) Three-dimensional black-blood t1-weighted turbo spin-echo techniques for the diagnosis of deep vein thrombosis in comparison with contrast-enhanced magnetic resonance imaging: a pilot study. Investig Radiol 50:401–408
Both M, Nolle B, von Forstner C, Moosig F, Gross WL, Heller M (2009) Imaging techniques in the evaluation of primary large vessel vasculitides: part 1: angiography, interventional therapy, and magnetic resonance imaging. Z Rheumatol 68:471–484
Brack A, Martinez-Taboada V, Stanson A, Goronzy JJ, Weyand CM (1999) Disease pattern in cranial and large-vessel giant cell arteritis. Arthritis Rheum 42:311–317
Aschwanden M, Daikeler T, Kesten F et al (2013) Temporal artery compression sign--a novel ultrasound finding for the diagnosis of giant cell arteritis. Ultraschall Med 34:47–50
Aschwanden M, Imfeld S, Staub D et al (2015) The ultrasound compression sign to diagnose temporal giant cell arteritis shows an excellent interobserver agreement. Clin Exp Rheumatol 33:S-113–S-115
Kermani TA, Warrington KJ (2013) Polymyalgia rheumatica. Lancet 381:63–72
Wasserman BA, Wityk RJ, Trout HH 3rd, Virmani R (2005) Low-grade carotid stenosis: looking beyond the lumen with MRI. Stroke 36:2504–2513
Yamada K, Yoshimura S, Kawasaki M et al (2011) Embolic complications after carotid artery stenting or carotid endarterectomy are associated with tissue characteristics of carotid plaques evaluated by magnetic resonance imaging. Atherosclerosis 215:399–404
Norenberg D, Ebersberger HU, Diederichs G, Hamm B, Botnar RM, Makowski MR (2015) Molecular magnetic resonance imaging of atherosclerotic vessel wall disease. Eur Radiol. doi:10.1007/s00330-015-3881-2
Swartz RH, Bhuta SS, Farb RI et al (2009) Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced MRI. Neurology 72:627–634
Schmidt WA, Kraft HE, Vorpahl K, Volker L, Gromnica-Ihle EJ (1997) Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med 337:1336–1342
Bley TA, Markl M, Schelp M et al (2008) Mural inflammatory hyperenhancement in MRI of giant cell (temporal) arteritis resolves under corticosteroid treatment. Rheumatology (Oxford) 47:65–67
Veldhoen S, Klink T, Geiger J et al (2014) MRI displays involvement of the temporalis muscle and the deep temporal artery in patients with giant cell arteritis. Eur Radiol 24:2971–2979
Kuker W (2007) Cerebral vasculitis: imaging signs revisited. Neuroradiology 49:471–479
Kuker W, Gaertner S, Nagele T et al (2008) Vessel wall contrast enhancement: a diagnostic sign of cerebral vasculitis. Cerebrovasc Dis 26:23–29
Besson FL, Parienti JJ, Bienvenu B et al (2011) Diagnostic performance of (1)(8)F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 38:1764–1772
Treglia G, Mattoli MV, Leccisotti L, Ferraccioli G, Giordano A (2011) Usefulness of whole-body fluorine-18-fluorodeoxyglucose positron emission tomography in patients with large-vessel vasculitis: a systematic review. Clin Rheumatol 30:1265–1275
de Leeuw K, Bijl M, Jager PL (2004) Additional value of positron emission tomography in diagnosis and follow-up of patients with large vessel vasculitides. Clin Exp Rheumatol 22:S21–S26
Li AE, Kamel I, Rando F et al (2004) Using MRI to assess aortic wall thickness in the multiethnic study of atherosclerosis: distribution by race, sex, and age. AJR Am J Roentgenol 182:593–597
Rosero EB, Peshock RM, Khera A, Clagett P, Lo H, Timaran CH (2011) Sex, race, and age distributions of mean aortic wall thickness in a multiethnic population-based sample. J Vasc Surg 53:950–957
Karassa FB, Matsagas MI, Schmidt WA, Ioannidis JP (2005) Meta-analysis: test performance of ultrasonography for giant-cell arteritis. Ann Intern Med 142:359–369
Salvarani C, Silingardi M, Ghirarduzzi A et al (2002) Is duplex ultrasonography useful for the diagnosis of giant-cell arteritis? Ann Intern Med 137:232–238
Acknowledgments
The scientific guarantor of this publication is Prof. Dr. Tobias Saam. The authors of this manuscript declare relationships with the following companies: Dr. Hendrik Kooijman-Kurfuerst is a physicist, who works for Philips Healthcare. He modified the original VISTA sequence and so developed the mVISTA sequence.
The other authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. One of the authors has significant statistical expertise. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Some study subjects or cohorts have been previously reported at the RSNA Meeting 2014 and at the ISMRM meeting 2015. Pdf-versions of the corresponding power-point presentations are attached as supplemental material. The RSNA-presentation reports about 14 patients and 14 controls of the entire study cohort. The ISMRM-presentation includes the entire study cohort of 70 subjects.
Methodology: prospective, case-control study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Treitl, K.M., Maurus, S., Sommer, N.N. et al. 3D-black-blood 3T-MRI for the diagnosis of thoracic large vessel vasculitis: A feasibility study. Eur Radiol 27, 2119–2128 (2017). https://doi.org/10.1007/s00330-016-4525-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4525-x