Abstract
Objectives
To characterize a radiogenomic risk score (RRS), a previously defined biomarker, and to evaluate its potential for stratifying radiological progression-free survival (rPFS) in patients with metastatic renal cell carcinoma (mRCC) undergoing pre-surgical treatment with bevacizumab.
Methodology
In this IRB-approved study, prospective imaging analysis of the RRS was performed on phase II clinical trial data of mRCC patients (n = 41) evaluating whether patient stratification according to the RRS resulted in groups more or less likely to have a rPFS to pre-surgical bevacizumab prior to cytoreductive nephrectomy. Survival times of RRS subgroups were analyzed using Kaplan-Meier survival analysis.
Results
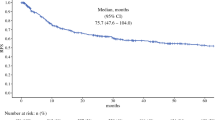
The RRS is enriched in diverse molecular processes including drug response, stress response, protein kinase regulation, and signal transduction pathways (P < 0.05). The RRS successfully stratified rPFS to bevacizumab based on pre-treatment computed tomography imaging with a median progression-free survival of 6 versus >25 months (P = 0.005) and overall survival of 25 versus >37 months in the high and low RRS groups (P = 0.03), respectively. Conventional prognostic predictors including the Motzer and Heng criteria were not predictive in this cohort (P > 0.05).
Conclusions
The RRS stratifies rPFS to bevacizumab in patients from a phase II clinical trial with mRCC undergoing cytoreductive nephrectomy and pre-surgical bevacizumab.
Key Points
• The RRS SOMA stratifies patient outcomes in a phase II clinical trial.
• RRS stratifies subjects into prognostic groups in a discrete or continuous fashion.
• RRS is biologically enriched in diverse processes including drug response programs.




Similar content being viewed by others
Abbreviations
- RRS:
-
radiogenomic risk score
- SOMA:
-
surrogate of molecular assay
- WHO:
-
World Health Organization
- SPC:
-
supervised principal component
- ccRCC:
-
clear cell renal cell carcinoma
- mRCC:
-
metastatic renal cell carcinoma
- GO:
-
gene ontology
- MSKCC:
-
Memorial Sloan Kettering Cancer Center
- RECIST:
-
response evaluation criteria in solid tumours
References
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ (2007) Cancer statistics, 2007. CA Cancer J Clin 57:43–66
Kuo MD, Gollub J, Sirlin CB, Ooi C, Chen X (2007) Radiogenomic analysis to identify imaging phenotypes associated with drug response gene expression programs in hepatocellular carcinoma. J Vasc Interv Radiol 18:821–831
Kuo MD, Jamshidi N (2014) Behind the numbers: decoding molecular phenotypes with radiogenomics--guiding principles and technical considerations. Radiology 270:320–325
Banerjee S, Wang DS, Kim HJ et al (2015) A computed tomography radiogenomic biomarker predicts microvascular invasion and clinical outcomes in hepatocellular carcinoma. Hepatology. doi:10.1002/hep.27877
Diehn M, Nardini C, Wang DS et al (2008) Identification of noninvasive imaging surrogates for brain tumor gene-expression modules. Proc Natl Acad Sci U S A 105:5213–5218
Jamshidi N, Diehn M, Bredel M, Kuo MD (2014) Illuminating radiogenomic characteristics of glioblastoma multiforme through integration of MR imaging, messenger RNA expression, and DNA copy number variation. Radiology 270:1–2
Segal E, Sirlin CB, Ooi C et al (2007) Decoding global gene expression programs in liver cancer by noninvasive imaging. Nat Biotechnol 25:675–680
Yamamoto S, Han W, Kim Y et al (2015) Breast cancer: radiogenomic biomarker reveals associations among dynamic contrast-enhanced mr imaging, long noncoding RNA, and metastasis. Radiology. doi:10.1148/radiol.15142698:142698
Yamamoto S, Korn RL, Oklu R et al (2014) ALK molecular phenotype in non-small cell lung cancer: CT radiogenomic characterization. Radiology 272:568–576
Gevaert O, Xu J, Hoang CD et al (2012) Non-small cell lung cancer: identifying prognostic imaging biomarkers by leveraging public gene expression microarray data--methods and preliminary results. Radiology 264:387–396
Rizzo S, Petrella F, Buscarino V et al (2015) CT radiogenomic characterization of EGFR, K-RAS, and ALK mutations in non-small cell lung cancer. Eur Radiol. doi:10.1007/s00330-015-3814-0
Klein EA, Cooperberg MR, Magi-Galluzzi C et al (2014) A 17-gene assay to predict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. Eur Urol 66:550–560
Sestak I, Dowsett M, Zabaglo L et al (2013) Factors predicting late recurrence for estrogen receptor-positive breast cancer. J Natl Cancer Inst 105:1504–1511
Solomon BJ, Mok T, Kim DW et al (2014) First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 371:2167–2177
Jamshidi N, Jonasch E, Zapala M et al (2015) The radiogenomic risk score: construction of a prognostic quantitative, noninvasive image-based molecular assay for renal cell carcinoma. Radiology 277:114–123
Jonasch E, Wood CG, Matin SF et al (2009) Phase II presurgical feasibility study of bevacizumab in untreated patients with metastatic renal cell carcinoma. J Clin Oncol 27:4076–4081
Sun M, Shariat SF, Cheng C et al (2011) Prognostic factors and predictive models in renal cell carcinoma: a contemporary review. Eur Urol 60:644–661
Heng DY, Xie W, Regan MM et al (2013) External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol 14:141–148
Heng DY, Xie W, Regan MM et al (2009) Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 27:5794–5799
Motzer RJ, Escudier B, Bukowski R et al (2013) Prognostic factors for survival in 1059 patients treated with sunitinib for metastatic renal cell carcinoma. Br J Cancer 108:2470–2477
Zeeberg BR, Feng W, Wang G et al (2003) GoMiner: a resource for biological interpretation of genomic and proteomic data. Genome Biol 4:R28
Breslow N, Crowley J (1984) A large sample study of the life table and product limit estimates under random censorship. Ann Stat 2:437–453
Viera AJ, Garrett JM (2005) Understanding interobserver agreement: the kappa statistic. Fam Med 37:360–363
Mekhail TM, Abou-Jawde RM, Boumerhi G et al (2005) Validation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol 23:832–841
Finn RS, Crown JP, Lang I et al (2015) The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 16:25–35
Robert C, Long GV, Brady B et al (2015) Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372:320–330
Lamuraglia M, Raslan S, Elaidi R et al (2015) mTOR-inhibitor treatment of metastatic renal cell carcinoma: contribution of Choi and modified Choi criteria assessed in 2D or 3D to evaluate tumor response. Eur Radiol. doi:10.1007/s00330-015-3828-7
Gerlinger M, Rowan AJ, Horswell S et al (2012) Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 366:883–892
Lindstrom LS, Karlsson E, Wilking UM et al (2012) Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol 30:2601–2608
Arrowsmith J (2011) Trial watch: phase II failures: 2008-2010. Nat Rev Drug Discov 10:328–329
Coffey CS, Levin B, Clark C et al (2012) Overview, hurdles, and future work in adaptive designs: perspectives from a National Institutes of Health-funded workshop. Clin Trials 9:671–680
Ledford H (2013) ‘Master protocol’ aims to revamp cancer trials. Nature 498:146–147
Acknowledgments
MDK is the scientific guarantor of this publication. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. MZ, NJ, and MDK have significant statistical expertise. Institutional review board approval was obtained from Umea Hospital and the MD Anderson Cancer Center. Written informed consent was obtained from all subjects in this study. None of the study cohort imaging findings or results have been previously reported in the literature. This is a retrospective, diagnostic/prognostic, single institution study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors disclose no potential conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 366 kb)
Rights and permissions
About this article
Cite this article
Jamshidi, N., Jonasch, E., Zapala, M. et al. The radiogenomic risk score stratifies outcomes in a renal cell cancer phase 2 clinical trial. Eur Radiol 26, 2798–2807 (2016). https://doi.org/10.1007/s00330-015-4082-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-4082-8