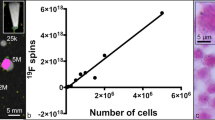
Abstract
19F MRI is emerging as a new imaging technique for cell tracking. It is particularly attractive because of its potential for direct and precise cell quantification. The most important challenge towards in vivo applications is the sensitivity of the technique, i.e. the detection limit in a reasonable imaging time. Optimal sensitivity can be achieved with dedicated 19F compounds together with specifically adapted hardware and acquisition methods. In this paper we introduce the 19F MRI technique focusing on these key sensitivity issues and review the state-of-the-art of 19F MRI and developments towards its clinical use. We calculate 19F detection limits reported in preclinical cell and clinical 19F drug studies in terms of tissue concentration in a 1 cm3 voxel, as an alternate way to compare detection limits. We estimate that a tissue concentration of a few millimoles per litre (mM) of 19F is required for a human study at a resolution of 1 cm3.
Key Points
• Direct and precise cell quantification can be done by 19 F MRI.
• 19 F MRI sensitivity is the most important parameter towards clinical application.
• A number of (technical) considerations can improve sensitivity significantly.
• A few millimoles per litre (mM) of 19 F per voxel is required for adequate detection.



Similar content being viewed by others
Abbreviations
- CA:
-
Contrast agent
- 2D:
-
Two-dimensional
- Dy:
-
Dysprosium
- FDA:
-
Food and drug administration
- FOV:
-
Field of view
- F-uTSI:
-
Fluorine ultrafast turbo spectroscopic imaging
- GRE:
-
Gradient echo
- MRI:
-
Magnetic resonance imaging
- MRSI:
-
Magnetic resonance spectroscopic imaging
- PFC:
-
Perfluorocarbon
- PFOB:
-
Perfluorooctyl bromide
- RF:
-
Radiofrequency
- ROI:
-
Region of interest
- SAR:
-
Specific absorption rate
- SE:
-
Spin echo
- SNR/t:
-
Signal to noise ratio per unit scan time
- SSFP:
-
Steady-state free precession
- TE:
-
Echo time
- TR:
-
Repetition time
- UTE:
-
Ultrashort echo time
- ZTE:
-
Zero echo time
References
Ahrens ET, Bulte JW (2013) Tracking immune cells in vivo using magnetic resonance imaging. Nat Rev Immunol 13:755–763
Laniado M, Weinmann HJ, Schorner W, Felix R, Speck U (1984) First use of GdDTPA/dimeglumine in man. Physiol Chem Phys Med NMR 16:157–165
Weinmann HJ, Brasch RC, Press WR, Wesbey GE (1984) Characteristics of gadolinium-DTPA complex: a potential NMR contrast agent. Ajr 142:619–624
Amiri H, Bustamante R, Millan A et al (2011) Magnetic and relaxation properties of multifunctional polymer-based nanostructured bioferrofluids as MRI contrast agents. Magn Reson Med 66:1715–1721
Amiri H, Mahmoudi M, Lascialfari A (2011) Superparamagnetic colloidal nanocrystal clusters coated with polyethylene glycol fumarate: a possible novel theranostic agent. Nanoscale 3:1022–1030
Economopoulos V, Chen Y, McFadden C, Foster PJ (2013) MRI detection of nonproliferative tumor cells in lymph node metastases using iron oxide particles in a mouse model of breast cancer. Transl Oncol 6:347–354
Odintsov B, Chun JL, Berry SE (2013) Whole body MRI and fluorescent microscopy for detection of stem cells labeled with superparamagnetic iron oxide (SPIO) nanoparticles and DiI following intramuscular and systemic delivery. Methods Mol Biol 1052:1–17
Lind K, Kresse M, Debus NP, Muller RH (2002) A novel formulation for superparamagnetic iron oxide (SPIO) particles enhancing MR lymphography: comparison of physicochemical properties and the in vivo behaviour. J Drug Target 10:221–230
Mack MG, Balzer JO, Straub R, Eichler K, Vogl TJ (2002) Superparamagnetic iron oxide-enhanced MR imaging of head and neck lymph nodes. Radiology 222:239–244
Weissleder R, Elizondo G, Wittenberg J, Lee AS, Josephson L, Brady TJ (1990) Ultrasmall superparamagnetic iron oxide: an intravenous contrast agent for assessing lymph nodes with MR imaging. Radiology 175:494–498
Larsen EK, Nielsen T, Wittenborn T et al (2012) Accumulation of magnetic iron oxide nanoparticles coated with variably sized polyethylene glycol in murine tumors. Nanoscale 4:2352–2361
Poselt E, Schmidtke C, Fischer S et al (2012) Tailor-made quantum dot and iron oxide based contrast agents for in vitro and in vivo tumor imaging. ACS Nano 6:3346–3355
Zimmer C, Weissleder R, Poss K, Bogdanova A, Wright SC Jr, Enochs WS (1995) MR imaging of phagocytosis in experimental gliomas. Radiology 197:533–538
Boulland JL, Leung DS, Thuen M et al (2012) Evaluation of intracellular labeling with micron-sized particles of iron oxide (MPIOs) as a general tool for in vitro and in vivo tracking of human stem and progenitor cells. Cell Transplant 21:1743–1759
Frank JA, Miller BR, Arbab AS et al (2003) Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology 228:480–487
Himmelreich U, Dresselaers T (2009) Cell labeling and tracking for experimental models using magnetic resonance imaging. Methods (San Diego, Calif) 48:112–124
Ahrens ET, Zhong J (2013) In vivo MRI cell tracking using perfluorocarbon probes and fluorine-19 detection. NMR Biomed 26:860–871
Bulte JW, Walczak P, Gleich B et al (2011) MPI cell tracking: what can we learn from MRI? Proc Soc Photo-Optical Instrum Eng 7965:79650z
Srinivas M, Heerschap A, Ahrens ET, Figdor CG, de Vries IJ (2010) (19)F MRI for quantitative in vivo cell tracking. Trends Biotechnol 28:363–370
Ruiz-Cabello J, Walczak P, Kedziorek DA et al (2008) In vivo “hot spot” MR imaging of neural stem cells using fluorinated nanoparticles. Magn Reson Med 60:1506–1511
Srinivas M, Boehm-Sturm P, Figdor CG, de Vries IJ, Hoehn M (2012) Labeling cells for in vivo tracking using (19)F MRI. Biomaterials 33:8830–8840
Waters EA, Chen J, Allen JS, Zhang H, Lanza GM, Wickline SA (2008) Detection and quantification of angiogenesis in experimental valve disease with integrin-targeted nanoparticles and 19-fluorine MRI/MRS. J Cardiovasc Magn Reson 10:43
Boehm-Sturm P, Mengler L, Wecker S, Hoehn M, Kallur T (2011) In vivo tracking of human neural stem cells with 19F magnetic resonance imaging. PLoS One 6:e29040
Maki J, Masuda C, Morikawa S et al (2007) The MR tracking of transplanted ATDC5 cells using fluorinated poly-L-lysine-CF3. Biomaterials 28:434–440
Hitchens TK, Ye Q, Eytan DF, Janjic JM, Ahrens ET, Ho C (2011) 19F MRI detection of acute allograft rejection with in vivo perfluorocarbon labeling of immune cells. Magn Reson Med 65:1144–1153
Flogel U, Ding Z, Hardung H et al (2008) In vivo monitoring of inflammation after cardiac and cerebral ischemia by fluorine magnetic resonance imaging. Circulation 118:140–148
Jacoby C, Temme S, Mayenfels F et al (2014) Probing different perfluorocarbons for in vivo inflammation imaging by 19F MRI: image reconstruction, biological half-lives and sensitivity. NMR Biomed 27:261–271
Majcher K, Tomanek B, Jasinski A et al (2006) Simultaneous functional magnetic resonance imaging in the rat spinal cord and brain. Exp Neurol 197:458–464
Srinivas M, Cruz LJ, Bonetto F, Heerschap A, Figdor CG, de Vries IJ (2010) Customizable, multi-functional fluorocarbon nanoparticles for quantitative in vivo imaging using 19F MRI and optical imaging. Biomaterials 31:7070–7077
Moser E, Stahlberg F, Ladd ME, Trattnig S (2012) 7-T MR–from research to clinical applications? NMR Biomed 25:695–716
Chen W, Takahashi A, Han E (2011) Quantitative T(1)(rho) imaging using phase cycling for B0 and B1 field inhomogeneity compensation. Magn Reson Imaging 29:608–619
Tannus A, Garwood M (1997) Adiabatic pulses. NMR Biomed 10:423–434
Watanabe H, Takaya N, Mitsumori F (2011) Non-uniformity correction of human brain imaging at high field by RF field mapping of B1+ and B1. J Magn Reson 212:426–430
Hockett FD, Wallace KD, Schmieder AH et al (2011) Simultaneous dual frequency 1H and 19F open coil imaging of arthritic rabbit knee at 3T. IEEE Trans Med Imaging 30:22–27
Wang C, Li Y, Wu B et al (2012) A practical multinuclear transceiver volume coil for in vivo MRI/MRS at 7 T. Magn Reson Imaging 30:78–84
Waiczies H, Lepore S, Drechsler S et al (2013) Visualizing brain inflammation with a shingled-leg radio-frequency head probe for (19)f/(1)h MRI. Sci Rep 3:1280
Hu L, Hockett FD, Chen J et al (2011) A generalized strategy for designing (19)F/(1)H dual-frequency MRI coil for small animal imaging at 4.7 Tesla. J Magn Reson Imaging 34:245–252
Roemer PB, Edelstein WA, Hayes CE, Souza SP, Mueller OM (1990) The NMR phased array. Magn Reson Med 16:192–225
McRobbie DW, Moore EA, Graves MJ, Prince MR (2007) MRI from picture to proton. Press, Cambridge University
Giraudeau C, Flament J, Marty B et al (2010) A new paradigm for high-sensitivity 19F magnetic resonance imaging of perfluorooctylbromide. Magn Reson Med 63:1119–1124
Chalmers KH, De Luca E, Hogg NH et al (2010) Design principles and theory of paramagnetic fluorine-labelled lanthanide complexes as probes for (19)F magnetic resonance: a proof-of-concept study. Chemistry (Weinheim an der Bergstrasse, Germany) 16:134–148
Chalmers KH, Kenwright AM, Parker D, Blamire AM (2011) 19F-lanthanide complexes with increased sensitivity for 19F-MRI: optimization of the MR acquisition. Magn Reson Med 66:931–936
Bonetto F, Srinivas M, Heerschap A et al (2011) A novel (19)F agent for detection and quantification of human dendritic cells using magnetic resonance imaging. Int J Cancer 129:365–373
Schmid F, Holtke C, Parker D, Faber C (2012) Boosting (19) F MRI-SNR efficient detection of paramagnetic contrast agents using ultrafast sequences. Magn Reson Med
Yildirim M, Keupp J, Lamerichs R (2007) Chemical shift independent imaging of 19F contrast agents using ultrafast MRSI (F-uTSI). Proc Int Soc Magn Reson Med 15:1249
Yildirim M, Díaz-López R, Nicolay K, Rolf L (2013) In vivo 3D spectroscopic imaging of 19F compounds using backprojection. Proc Int Soc Magn Reson Med 21:4009
Hertlein T, Sturm V, Kircher S et al (2011) Visualization of abscess formation in a murine thigh infection model of Staphylococcus aureus by 19F-magnetic resonance imaging (MRI). PLoS One 6:e18246
Srinivas M, Bonetto F, Tel J, et al (2012) Simultaneous and quantitative tracking of distinct cell populations using 19F MRI(ed)^(eds) ISMRM. Intl Soc Mag Reson Med, Melbourne, Australia pp 4370
Zhong J, Mills PH, Hitchens TK, Ahrens ET (2013) Accelerated fluorine-19 MRI cell tracking using compressed sensing. Magn Reson Med 69:1683–1690
Goette MJ, Keupp J, Rahmer J, Lanza GM, Wickline SA, Caruthers SD (2014) Balanced UTE-SSFP for F MR imaging of complex spectra. Magn Reson Med
Ahrens ET, Flores R, Xu H, Morel PA (2005) In vivo imaging platform for tracking immunotherapeutic cells. Nat Biotechnol 23:983–987
Srinivas M, Morel PA, Ernst LA, Laidlaw DH, Ahrens ET (2007) Fluorine-19 MRI for visualization and quantification of cell migration in a diabetes model. Magn Reson Med 58:725–734
Kok MB, de Vries A, Abdurrachim D et al (2011) Quantitative (1)H MRI, (19)F MRI, and (19)F MRS of cell-internalized perfluorocarbon paramagnetic nanoparticles. Contrast Media Mol Imaging 6:19–27
van Heeswijk RB, De Blois J, Kania G et al (2013) Selective in vivo visualization of immune-cell infiltration in a mouse model of autoimmune myocarditis by fluorine-19 cardiac magnetic resonance. Circulation 6:277–284
Klomp D, van Laarhoven H, Scheenen T, Kamm Y, Heerschap A (2007) Quantitative 19F MR spectroscopy at 3 T to detect heterogeneous capecitabine metabolism in human liver. NMR Biomed 20:485–492
Lee CP, Payne GS, Oregioni A et al (2009) A phase I study of the nitroimidazole hypoxia marker SR4554 using 19F magnetic resonance spectroscopy. Br J Cancer 101:1860–1868
Kadayakkara DK, Ranganathan S, Young WB, Ahrens ET (2012) Assaying macrophage activity in a murine model of inflammatory bowel disease using fluorine-19 MRI. Lab Investig J Tech Methods Pathol 92:636–645
Srinivas M, Turner MS, Janjic JM, Morel PA, Laidlaw DH, Ahrens ET (2009) In vivo cytometry of antigen-specific t cells using 19F MRI. Magn Reson Med 62:747–753
Ahrens ET, Young WB, Xu H, Pusateri LK (2011) Rapid quantification of inflammation in tissue samples using perfluorocarbon emulsion and fluorine-19 nuclear magnetic resonance. BioTechniques 50:229–234
Stoll G, Basse-Lusebrink T, Weise G, Jakob P (2012) Visualization of inflammation using (19) F-magnetic resonance imaging and perfluorocarbons. Wiley Interdiscip Rev 4:438–447
Temme S, Bonner F, Schrader J, Flogel U (2012) 19F magnetic resonance imaging of endogenous macrophages in inflammation. Wiley Interdiscip Rev 4:329–343
Weise G, Basse-Luesebrink TC, Wessig C, Jakob PM, Stoll G (2011) In vivo imaging of inflammation in the peripheral nervous system by (19)F MRI. Exp Neurol 229:494–501
Flogel U, Su S, Kreideweiss I et al (2011) Noninvasive detection of graft rejection by in vivo (19) F MRI in the early stage. Am J Transplant 11:235–244
Zarif L, Postel M, Trevino L, Riess JG, Valla A, Follana R (1994) Biodistribution and excretion of a mixed fluorocarbon-hydrocarbon “dowel” emulsion as determined by 19F NMR. Artif Cells Blood Substit Immobil Biotechnol 22:1193–1198
de Vries IJ, Lesterhuis WJ, Barentsz JO et al (2005) Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol 23:1407–1413
Tirotta I, Mastropietro A, Cordiglieri C et al (2014) A superfluorinated molecular probe for highly sensitive in vivo (19)F-MRI. J Am Chem Soc 136:8524–8527
Ahrens ET, Balducci A, Helfer B, et al (2014) First clinical experience using fluorine-19 MRI to track immunotherapeutic dendritic cells in colorectal cancer patients(ed)^(eds) ISMRM. Intl Soc Mag Reson Med, Milan, Italy, pp 0474
Acknowledgements
The scientific guarantor of this publication is Prof. Arend Heerschap. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. This work was financially supported by the European Union EU-FP7 ENCITE (HEALTH-F5-2008-201842) grant and Netherlands Institute for Regenerative Medicine (NIRM) FES0908. MS is supported by the Netherlands Organization for Scientific Research (NWO) VENI 700.10.409 and the European Research Council (ERC) ERC-2014-StG-336454-CoNQUeST and JdV by NWO-VIDI 917.76.363. Methodology: performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amiri, H., Srinivas, M., Veltien, A. et al. Cell tracking using 19F magnetic resonance imaging: Technical aspects and challenges towards clinical applications. Eur Radiol 25, 726–735 (2015). https://doi.org/10.1007/s00330-014-3474-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3474-5