Abstract
Objectives
Low-grade cerebral oedema is considered to be pathognomonic of minimal hepatic encephalopathy (MHE) in cirrhotic patients. The purpose of this study was to investigate both the grey matter (GM) and white matter (WM) changes in a homogeneous cohort of patients with MHE by combining voxel-based morphometry (VBM) and tract-based spatial statistics (TBSS).
Methods
Twenty-five MHE patients and 25 healthy controls participated in the study with three-dimensional T1 and diffusion-tensor imaging. Group differences in regional GM volume were assessed using VBM analysis while differences in fractional anisotropy (FA), mean diffusivity (MD) of WM were compared using TBSS analysis.
Results
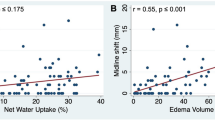
VBM displayed extensively decreased GM volume in MHE, mainly located in the frontal and temporal cortices, paracentral lobule, caudate, putamen and amygdale, and increased GM volume in the thalamus. TBSS showed decreased FA in MHE patients in the corpus callosum, cingulum, internal/external capsule, corticospinal tract, superior longitudinal fasciculus and posterior corona radiata. Areas of increased MD in MHE patients were more extensive and included, in addition to all the areas of decreased FA, the anterior corona radiata, inferior fronto-occipital fasciculus, fornix and the middle cerebellar peduncle.
Conclusion
The results suggest that cortical atrophy and low-grade brain oedema in WM co-exist in MHE.
Key Points
• Minimal hepatic encephalopathy develops before major neuropathological destruction occurs.
• Cortical atrophy and low-grade brain oedema of white matter co-exist in MHE.
• Blood ammonia correlates with abnormal WM indices in MHE patients.
• Imaging findings could assist decisions about therapy in patients with cirrhosis.



Similar content being viewed by others
References
Ortiz M, Jacas C, Cordoba J (2005) Minimal hepatic encephalopathy: diagnosis, clinical significance and recommendations. J Hepatol 42:S45–S53
Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT (2002) Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 35:716–721
Groeneweg M, Quero JC, De Bruijn I et al (1998) Subclinical hepatic encephalopathy impairs daily functioning. Hepatology 28:45–49
Bajaj JS, Hafeezullah M, Hoffmann RG et al (2008) Navigation skill impairment: another dimension of the driving difficulties in minimal hepatic encephalopathy. Hepatology 47:596–604
Schomerus H, Schreiegg J (1993) Prevalence of latent portasystemic encephalopathy in an unselected population of patients with liver cirrhosis in general practice. Z Gastroenterol 31:231–234
Moore JW, Dunk AA, Crawford JR et al (1989) Neuropsychological deficits and morphological MRI brain scan abnormalities in apparently healthy non-encephalopathic patients with cirrhosis. A controlled study. J Hepatol 9:319–325
Jover R, Rodrigo R, Felipo V et al (2006) Brain edema and inflammatory activation in bile duct ligated rats with diet-induced hyperammonemia: a model of hepatic encephalopathy in cirrhosis. Hepatology 43:1257–1266
Rivera-Mancia S, Rios C, Montes S (2012) Manganese and ammonia interactions in the brain of cirrhotic rats: effects on brain ammonia metabolism. Neurochem Res 37:1074–1084
Zhang LJ, Zhong J, Lu GM (2013) Multimodality MR imaging findings of low-grade brain edema in Hepatic encephalopathy. AJNR Am J Neuroradiol 34:707–715
Kale RA, Gupta RK, Saraswat VA et al (2006) Demonstration of interstitial cerebral edema with diffusion tensor MR imaging in type C hepatic encephalopathy. Hepatology 43:698–706
Mardini H, Smith FE, Record CO, Blamire AM (2011) Magnetic resonance quantification of water and metabolites in the brain of cirrhotics following induced hyperammonaemia. J Hepatol 54:1154–1160
Prakash R, Mullen KD (2010) Mechanisms, diagnosis and management of hepatic encephalopathy. Nat Rev Gastroenterol Hepatol 7:515–525
Ashburner J, Friston KJ (2000) Voxel-based morphometry—the methods. NeuroImage 11:805–821
Chen HJ, Zhu XQ, Shu H et al (2012) Structural and functional cerebral impairments in cirrhotic patients with a history of overt hepatic encephalopathy. Eur J Radiol 81:2463–2469
Iwasa M, Mifuji-Moroka R, Kuroda M et al (2012) Regional reduction in gray and white matter volume in brains of cirrhotic patients: voxel-based analysis of MRI. Metab Brain Dis 27:551–557
Smith SM, Jenkinson M, Johansen-Berg H et al (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage 31:1487–1505
Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS (2001) A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage 14:21–36
Mechelli A, Price CJ, Friston KJ, Ashburner J (2005) Voxel-based morphometry of the human brain: methods and applications. Curr Med Imaging Rev 1:105–113
Weissenborn K, Ruckert N, Hecker H, Manns MP (1998) The number connection tests A and B: interindividual variability and use for the assessment of early hepatic encephalopathy. J Hepatol 28:646–653
Weissenborn K, Ennen JC, Schomerus H, Ruckert N, Hecker H (2001) Neuropsychological characterization of hepatic encephalopathy. J Hepatol 34:768–773
Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R (1973) Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 60:646–649
Bitter T, Bruderle J, Gudziol H, Burmeister HP, Gaser C, Guntinas-Lichius O (2010) Gray program for New Century Excellent Talents in University (NCET-12-0260 to Long Jiang Zhang) and white matter reduction in hyposmic subjects—a voxel-based morphometry study. Brain Res 1347:42–47
Della Nave R, Ginestroni A, Tessa C et al (2010) Regional distribution and clinical correlates of white matter structural damage in Huntington disease: a tract-based spatial statistics study. AJNR Am J Neuroradiol 31:1675–1681
Agosta F, Pievani M, Sala S et al (2011) White matter damage in Alzheimer disease and its relationship to gray matter atrophy. Radiology 258:853–863
Smith SM, Nichols TE (2009) Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 44:83–98
Rai V, Nath K, Saraswat VA, Purwar A, Rathore RK, Gupta RK (2008) Measurement of cytotoxic and interstitial components of cerebral edema in acute hepatic failure by diffusion tensor imaging. J Magn Reson Imaging 28:334–341
Nath K, Saraswat VA, Krishna YR et al (2008) Quantification of cerebral edema on diffusion tensor imaging in acute-on-chronic liver failure. NMR Biomed 21:713–722
Assaf Y, Pasternak O (2008) Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci 34:51–61
Sugimoto R, Iwasa M, Maeda M et al (2008) Value of the apparent diffusion coefficient for quantification of low-grade hepatic encephalopathy. Am J Gastroenterol 103:1413–1420
Guevara M, Baccaro ME, Gomez-Anson B et al (2011) Cerebral magnetic resonance imaging reveals marked abnormalities of brain tissue density in patients with cirrhosis without overt hepatic encephalopathy. J Hepatol 55:564–573
Matsusue E, Kinoshita T, Ohama E, Ogawa T (2005) Cerebral cortical and white matter lesions in chronic hepatic encephalopathy: MR-pathologic correlations. AJNR Am J Neuroradiol 26:347–351
Herrero MT, Barcia C, Navarro JM (2002) Functional anatomy of thalamus and basal ganglia. Childs Nerv Syst 18:386–404
Lockwood AH, Weissenborn K, Bokemeyer M, Tietge U, Burchert W (2002) Correlations between cerebral glucose metabolism and neuropsychological test performance in nonalcoholic cirrhotics. Metab Brain Dis 17:29–40
Ahl B, Weissenborn K, van den Hoff J et al (2004) Regional differences in cerebral blood flow and cerebral ammonia metabolism in patients with cirrhosis. Hepatology 40:73–79
Giewekemeyer K, Berding G, Ahl B, Ennen JC, Weissenborn K (2007) Bradykinesia in cirrhotic patients with early hepatic encephalopathy is related to a decreased glucose uptake of frontomesial cortical areas relevant for movement initiation. J Hepatol 46:1034–1039
Norenberg MD (1987) The role of astrocytes in hepatic encephalopathy. Neurochem Pathol 6:13–33
Kumar R, Gupta RK, Elderkin-Thompson V et al (2008) Voxel-based diffusion tensor magnetic resonance imaging evaluation of low-grade hepatic encephalopathy. J Magn Reson Imaging 27:1061–1068
Poveda MJ, Bernabeu A, Concepcion L et al (2010) Brain edema dynamics in patients with overt hepatic encephalopathy A magnetic resonance imaging study. NeuroImage 52:481–487
Chavarria L, Alonso J, Garcia-Martinez R et al (2011) Biexponential analysis of diffusion-tensor imaging of the brain in patients with cirrhosis before and after liver transplantation. AJNR Am J Neuroradiol 32:1510–1517
Chen HJ, Wang Y, Zhu XQ, Cui Y, Chen YC, Teng GJ (2012) White matter abnormalities correlate with neurocognitive performance in patients with HBV-related cirrhosis. J Neurol Sci 321:65–72
Rovira A, Alonso J, Cordoba J (2008) MR imaging findings in hepatic encephalopathy. AJNR Am J Neuroradiol 29:1612–1621
Acknowledgements
This work was supported by the grants from the Natural Scientific Foundation of China (Grant Nos. 30700194 and 81230032 for Long Jiang Zhang, Grant No. 81101039 for Gang Zheng), program for New Century Excellent Talents in University (Grant Nos. NCET-12-0260 for Long Jiang Zhang) and Chinese Key Program (Grant Nos. BWS11J063 and 10z026 for Guang Ming Lu).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Qi, R., Zhang, L.J., Zhong, J. et al. Grey and white matter abnormalities in minimal hepatic encephalopathy: a study combining voxel-based morphometry and tract-based spatial statistics. Eur Radiol 23, 3370–3378 (2013). https://doi.org/10.1007/s00330-013-2963-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-013-2963-2