Abstract
Objectives
To evaluate whether changes in BOLD signal intensities following hyperoxygenation are related to intrauterine growth restriction (IUGR) in a rat model.
Methods
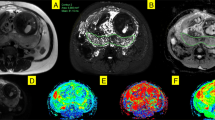
IUGR was induced in pregnant rats by ligating the left vascular uterine pedicle at day 16 of gestation. BOLD MR imaging using a balanced steady-state free-precession (balanced-SSFP) sequence on a 1.5-T system was performed on day 19. Signal intensities (SI) before and after maternal hyperoxygenation were compared in the maternal liver and in control and growth-restricted foetoplacental units (FPUs).
Results
Maternal hyperoxygenation resulted in a significant increase in SI in all regions of interest (P < 0.05) in the 18 rats. In the control group, the SI (mean ± SD) increased by 21 % ± 15 in placentas (n = 74) and 13 % ± 8.5 in foetuses (n = 53). In the IUGR group, the increase was significantly lower: 6.5 % ± 4 in placentas (n = 36) and 7 % ± 5.5 in foetuses (n = 34) (P < 0.05).
Conclusion
BOLD MRI allows non-invasive assessment of the foetoplacental response to maternal hyperoxygenation in the rat and demonstrates its alteration in an IUGR model. This imaging method may provide a useful adjunct for the early diagnosis, evaluation, and management of human IUGR.
Key Points
• Intra-uterine growth restriction is an important cause of perinatal morbidity and mortality.
• Blood oxygen level-dependent MRI non-invasively assesses foetoplacental response to maternal hyperoxygenation.
• In the rat, foetoplacental response to maternal hyperoxygenation is altered in IUGR.
• Functional MRI may help to assess human IUGR.



Similar content being viewed by others
Abbreviations
- IUGR:
-
Intra-uterine growth restriction
- FPU:
-
Foetoplacental unit
- BOLD:
-
Blood oxygen level dependent
- SI:
-
Signal intensity
References
Malassine A (2001) Morphological variability and placental function. Gynecol Obstet Fertil 29:489–496
Sibai BM, Lindheimer M, Hauth J et al (1998) Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med 339:667–671
Tsatsaris V, Fournier T, Winer N (2008) Pathophysiology of preeclampsia. J Gynecol Obstet Biol Reprod (Paris) 37:16–23
Salomon LJ, Siauve N, Balvay D, Cuenod CA, Vayssettes C, Luciani A et al (2005) Placental perfusion MR imaging with contrast agents in a mouse model. Radiology 235:73–80
Salomon LJ, Siauve N, Taillieu F et al (2006) In vivo dynamic MRI measurement of the noradrenaline-induced reduction in placental blood flow in mice. Placenta 27:1007–1013
Taillieu F, Salomon LJ, Siauve N et al (2006) Placental perfusion and permeability: simultaneous assessment with dual-echo contrast-enhanced MR imaging in mice. Radiology 241:737–745
Gore JC (2003) Principles and practice of functional MRI of the human brain. J Clin Invest 112:4–9
Le Bihan D, Lehericy S (1999) Practical aspects of realization of a functional MRI. J Neuroradiol 26:S54–S58
Rhee TK, Larson AC, Prasad PV et al (2005) Feasibility of blood oxygenation level-dependent MR imaging to monitor hepatic transcatheter arterial embolization in rabbits. J Vasc Interv Radiol 16:1523–1528
Ledermann HP, Heidecker HG, Schulte AC et al (2006) Calf muscles imaged at BOLD MR: correlation with TcPO2 and flowmetry measurements during ischemia and reactive hyperemia–initial experience. Radiology 241:477–484
Landuyt W, Hermans R, Bosmans H et al (2001) BOLD contrast fMRI of whole rodent tumour during air or carbogen breathing using echo-planar imaging at 1.5 T. Eur Radiol 11:2332–2340
Vincent K, Moore J, Kennedy S, Tracey I (2008) Blood oxygenation level dependent functional magnetic resonance imaging: current and potential uses in obstetrics and gynaecology. BJOG 116:240–246
Rodesch F, Simon P, Donner C, Jauniaux E (1992) Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol 80:283–285
Jauniaux E, Gulbis B, Burton GJ (2003) Physiological implications of the materno-fetal oxygen gradient in human early pregnancy. Reprod Biomed Online 7:250–253
Longo LD (1988) Transplacental gas exchange. Rev Mal Respir 5:197–206
Nicolaides KH, Economides DL, Soothill PW (1989) Blood gases, pH, and lactate in appropriate- and small-for-gestational-age fetuses. Am J Obstet Gynecol 161:996–1001
Soothill PW, Nicolaides KH, Bilardo CM, Campbell S (1986) Relation of fetal hypoxia in growth retardation to mean blood velocity in the fetal aorta. Lancet 2:1118–1120
Wilson RD, Farquharson DF, Wittmann BK, Shaw D (1994) Cordocentesis: overall pregnancy loss rate as important as procedure loss rate. Fetal Diagn Ther 9:142–148, Review
Delmaire C, Krainik A, Lethuc V et al (2007) Functional magnetic resonance imaging: physiopathology, techniques and applications. J Radiol 88:497–509
Habas C (2002) Physiological basis of functional MRI. J Radiol 83:1737–1741
Girsh E, Plaks V, Gilad AA et al (2007) Cloprostenol, a prostaglandin F(2alpha) analog, induces hypoxia in rat placenta: BOLD contrast MRI. NMR Biomed 20:28–39
Wedegartner U, Kooijman H, Andreas T, Beindorff N, Hecher K, Adam G (2010) T2 and T2* measurements of fetal brain oxygenation during hypoxia with MRI at 3 T: correlation with fetal arterial blood oxygen saturation. Eur Radiol 20:121–127
Wedgartner U, Tchirikov M, Schäfer S, Priest AN, Kooijman H, Adam G, Schröder HJ (2006) Functional MR imaging: comparison of BOLD signal intensity changes in fetal organs with fetal and maternal oxyhemoglobin saturation during hypoxia in sheep. Radiology 238:872–880
Sørensen A, Pedersen M, Tietze A, Ottosen L, Duus L, Uldbjerg N (2009) BOLD MRI in sheep fetuses: a non-invasive method for measuring changes in tissue oxygenation. Ultrasound Obstet Gynecol 34:687–692
Semple SI, Wallis F, Haggarty P et al (2001) The measurement of fetal liver T(*)(2) in utero before and after maternal oxygen breathing: progress towards a non-invasive measurement of fetal oxygenation and placental function. Magn Reson Imaging 19:921–928
Morris DM, Ross JAS, McVicar A et al (2010) Changes in fetal liver T2* measurements by MRI in response to maternal oxygen breathing: application to diagnosing fœtal growth restriction. Physiol Meas 31:1137–1146
Barker DJ (1996) Growth in utero and coronary heart disease. Nutr Rev 54:S1–S7
Salle BL, Chatelain P, Nicolino M, Claris O (2001) Intrauterine growth retardation, its consequences in infancy, in the child and long term. Bull Acad Natl Med 185:1271–1276
Wigglesworth JS (1964) Experimental growth retardation in the fetal rat. J Pathol Bacteriol 88:1–13
Wigglesworth JS (1967) Pathological and experimental aspects of fetal growth retardation. Proc R Soc Med 60:879–881
Ogawa S, Lee TM, Kay AR, Tank DW (1990) Brain Magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A 87:9868–9872
Li D, Waight DJ, Wang Y (1998) In vivo correlation between blood T2* and oxygen saturation. J Magn Reson Imaging 8:1236–1239
Li D, Wang Y, Waight DJ (1998) Blood oxygen saturation assessment in vivo using T2* estimation. Magn Reson Med 39:685–690
Scheffler K, Seifritz E, Bilecen D et al (2001) Detection of BOLD changes by means of a frequency-sensitive trueFISP technique: preliminary results. NMR Biomed 14:490–496
Miller KL, Smith SM, Jezzard P, Pauly JM (2006) High-resolution FMRI at 1.5 T using balanced SSFP. Magn Reson Med 55:161–170
Miller KL, Hargreaves BA, Lee J, Ress D, deCharms RC, Pauly JM (2003) Functional brain imaging using a blood oxygenation sensitive steady state. Magn Reson Med 50:675–683
Wright KB, Klocke FJ, Deshpande VS et al (2001) Assessment of regional differences in myocardial blood flow using T2-weighted 3D BOLD imaging. Magn Reson Med 46:573–578
Dharmakumar R, Hong J, Brittain JH, Plewes DB, Wright GA (2005) Oxygen sensitive contrast in blood for steady state free precession imaging. Magn Reson Med 53:574–583
Dharmakumar R, Qi X, Hong J, Wright GA (2006) Detecting microcirculatory changes in blood oxygen state with steady-state free precession imaging. Magn Reson Med 55:1372–1380
Vöhringer M, Flewitt JA, Green JD et al (2010) Oxygenation-sensitive CMR for assessing vasodilatator-induced changes of myocardial oxygenation. J Cardiovasc Magn Reson 31:12–20
Battaglia C, Artini PG, D’Ambrogio G, Galli PA, Segre A, Genazzani AR (1992) Maternal hyperoxygenation in the treatment of intrauterine growth retardation. Am J Obstet Gynecol 167:430–435
Say L, Gülmezoglu AM, Hofmeyr GJ (2003) Maternal oxygen administration for suspected impaired fetal growth. Cochrane Database Syst Rev: CD000137. doi:10.1002/14651858.CD000137
Nicolaides KH, Campbell S, Bradley RJ, Bilardo CM, Soothill PW, Gibb D (1987) Maternal oxygen therapy for intrauterine growth retardation. Lancet 1:942–945
Figueroa R, Maulik D (2006) Prenatal therapy for fetal growth restriction. Clin Obstet Gynecol 49:308–319
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aimot-Macron, S., Salomon, L.J., Deloison, B. et al. In vivo MRI assessment of placental and foetal oxygenation changes in a rat model of growth restriction using blood oxygen level-dependent (BOLD) magnetic resonance imaging. Eur Radiol 23, 1335–1342 (2013). https://doi.org/10.1007/s00330-012-2712-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-012-2712-y