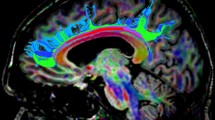
Abstract
Objectives
To assess the reliability of diffusion tensor imaging (DTI)-based fibre tractography (FT), which is a prerequisite for clinical applications of this technique. Here we assess the test–retest reproducibility of the architectural and microstructural features of two clinically relevant tracts reconstructed with DTI-FT.
Methods
The corticospinal tract (CST), arcuate fasciculus (AF) and its long segment (AFl) were reconstructed in 17 healthy subjects imaged twice using a deterministic approach. Coefficients of variation (CVs) of diffusion-derived tract values were used to assess the microstructural reproducibility. Spatial correlation and fibre overlap were used to assess the architectural reproducibility.
Results
Spatial correlation was 68 % for the CST and AF, and 69 % for the AFl. Overlap was 69 % for the CST, 61 % for the AF, and 59 % for the AFl. This was comparable to 2-mm tract shift variability. CVs of diffusion-derived tract values were at most 3.4 %.
Conclusions
The results showed low architectural and microstructural variability for the reconstruction of the tracts. The architectural reproducibility results encourage the further investigation of the use of DTI-FT for neurosurgical planning. The high microstructural reproducibility results are promising for using DTI-FT in neurology to assess or predict functional recovery.
Key Points
• Magnetic resonance diffusion tensor fibre tractography is increasingly used in the neurosciences.
• The architectural reproducibility of fibre pathways can be up to 69 %.
• However the microstructural variability of fibre pathways is only 3.4 % at most.
• The architectural reproducibility results encourage the use of DTI-FT for neurosurgery.
• The microstructural reproducibility results support the use of DTI-FT in neurology.




Similar content being viewed by others
References
Mori S, van Zijl PC (2002) Fiber tracking: principles and strategies - a technical review. NMR Biomed 15:468–480
Tournier JD, Mori S, Leemans A (2011) Diffusion tensor imaging and beyond. Magn Reson Med 65:1532–1556
Bello L, Gambini A, Castellano A et al (2008) Motor and language DTI Fiber Tracking combined with intraoperative subcortical mapping for surgical removal of gliomas. NeuroImage 39:369–382
Kinoshita M, Yamada K, Hashimoto N et al (2005) Fiber-tracking does not accurately estimate size of fiber bundle in pathological condition: initial neurosurgical experience using neuronavigation and subcortical white matter stimulation. NeuroImage 25:424–429
Kunimatsu A, Aoki S, Masutani Y, Abe O, Mori H, Ohtomo K (2003) Three-dimensional white matter tractography by diffusion tensor imaging in ischaemic stroke involving the corticospinal tract. Neuroradiology 45:532–535
van der Aa NE, Leemans A, Northington FJ et al (2011) Does diffusion tensor imaging-based tractography at 3 months of age contribute to the prediction of motor outcome after perinatal arterial ischemic stroke? Stroke 42:3410–3414
van Hecke W, Nagels G, Leemans A, Vandervliet E, Sijbers J, Parizel PM (2010) Correlation of cognitive dysfunction and diffusion tensor MRI measures in patients with mild and moderate multiple sclerosis. J Magn Reson Imaging 31:1492–1498
Ciccarelli O, Catani M, Johansen-Berg H, Clark C, Thompson A (2008) Diffusion-based tractography in neurological disorders: concepts, applications, and future developments. Lancet Neurol 7:715–727
Makris N, Kennedy DN, McInerney S et al (2005) Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex 15:854–869
Gloor P (1997) The temporal lobe and the limbic system. Oxford University Press, New York
Kiernan JA (1998) Barr’s the human nervous system: an anatomical view point. Lippincott–Raven, Philadelphia
Echtalew C (1992) Correlative anatomy of the nervous system. Macmillan, New York
Dejerine J (1985) Anatomie des Centres Nerveux. Rueff et Cie, Paris
Nieuwenhuys RVJHC (1988) The human central nervous system. Springer-Verlag, Berlin
Petrides M, Pandya DN (1988) Association fiber pathways to the frontal cortex from the superior temporal region in the rhesus monkey. J Comp Neurol 273:52–66
Bernal B, Ardila A (2009) The role of the arcuate fasciculus in conduction aphasia. Brain 132:2309–2316
Benson DF, Sheremata WA, Bouchard R, Segarra JM, Price D, Geschwind N (1973) Conduction aphasia. A clinicopathological study. Arch Neurol 28:339–346
Mesulam MM (2001) Primary progressive aphasia. Ann Neurol 49:425–432
Catani M, Mesulam M (2008) The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex 44:953–961
Verhoeven JS, Rommel N, Prodi E et al (2011) Is there a common neuroanatomical substrate of language deficit between autism spectrum disorder and specific language impairment? Cereb Cortex. doi:10.1093/cercor/bhr292
Clark CA, Byrnes T (2010) DTI and tractography in neurosurgical planning. In: Jones DK (ed) Diffusion MRI: theory, methods and applications. Oxford University Press, New York
Nimsky C, Ganslandt O, Hastreiter P et al (2005) Intraoperative diffusion-tensor MR imaging: shifting of white matter tracts during neurosurgical procedures – initial experience. Radiology 234:218–225
Wheeler-Kingshott CA, Cercignani M (2009) About “axial” and “radial” diffusivities. Magn Reson Med 61:1255–1260
Ciccarelli O, Parker GJ, Toosy AT et al (2003) From diffusion tractography to quantitative white matter tract measures: a reproducibility study. NeuroImage 18:348–359
Heiervang E, Behrens TE, Mackay CE, Robson MD, Johansen-Berg H (2006) Between session reproducibility and between subject variability of diffusion MR and tractography measures. NeuroImage 33:867–877
Danielian LE, Iwata NK, Thomasson DM, Floeter MK (2010) Reliability of fiber tracking measurements in diffusion tensor imaging for longitudinal study. NeuroImage 49:1572–1580
Tensaouti F, Lahlou I, Clarisse P, Lotterie JA, Berry I (2011) Quantitative and reproducibility study of four tractography algorithms used in clinical routine. J Magn Reson Imaging 34:165–172
Catani M, Howard RJ, Pajevic S, Jones DK (2002) Virtual in vivo interactive dissection of white matter fasciculi in the human brain. NeuroImage 17:77–94
Mori S, Kaufmann WE, Davatzikos C et al (2002) Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magn Reson Med 47:215–223
Wang JY, Abdi H, Bakhadirov K, az-Arrastia R, Devous MD Sr (2012) A comprehensive reliability assessment of quantitative diffusion tensor tractography. NeuroImage 60:1127–1138
Catani M, Jones DK, Ffytche DH (2005) Perisylvian language networks of the human brain. Ann Neurol 57:8–16
Matsumoto R, Okada T, Mikuni N et al (2008) Hemispheric asymmetry of the arcuate fasciculus: a preliminary diffusion tensor tractography study in patients with unilateral language dominance defined by Wada test. J Neurol 255:1703–1711
Lebel C, Beaulieu C (2009) Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Hum Brain Mapp 30:3563–3573
Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A (2000) In vivo fiber tractography using DT-MRI data. Magn Reson Med 44:625–632
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Jones DK, Leemans A (2011) Diffusion tensor imaging. Methods Mol Biol 711:127–144
Leemans A, Jeurissen B, Sijbers J et al. (2009) Explore DTI: a graphical toolbox for processing, analyzing, visualizing diffusion MR data. In: 17th Annual Meeting Proc Intl Soc Mag Reson Med, Hawaii USA, pp 3537
Leemans A, Jones DK (2009) The B-matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med 61:1336–1349
Chang LC, Jones DK, Pierpaoli C (2005) RESTORE: robust estimation of tensors by outlier rejection. Magn Reson Med 53:1088–1095
Bland JM, Altman DG (1996) Measurement error proportional to the mean. BMJ 313:106
Raemaekers M, Vink M, Zandbelt B, van Wezel RJ, Kahn RS, Ramsey NF (2007) Test-retest reliability of fMRI activation during prosaccades and antisaccades. NeuroImage 36:532–542
Bernal B, Altman N (2010) The connectivity of the superior longitudinal fasciculus: a tractography DTI study. Magn Reson Imaging 28:217–225
Alexander AL, Hasan KM, Lazar M, Tsuruda JS, Parker DL (2001) Analysis of partial volume effects in diffusion-tensor MRI. Magn Reson Med 45:770–780
Vos SB, Jones DK, Viergever MA, Leemans A (2011) Partial volume effect as a hidden covariate in DTI analyses. NeuroImage 55:1566–1576
Takao H, Hayashi N, Kabasawa H, Ohtomo K (2012) Effect of scanner in longitudinal diffusion tensor imaging studies. Hum Brain Mapp 33:466–477
Takao H, Hayashi N, Ohtomo K (2011) Effect of scanner in asymmetry studies using diffusion tensor imaging. NeuroImage 54:1053–1062
Jaermann T, Crelier G, Pruessmann KP et al (2004) SENSE-DTI at 3 T. Magn Reson Med 51:230–236
Jeurissen B, Leemans A, Jones DK, Tournier JD, Sijbers J (2011) Probabilistic fiber tracking using the residual bootstrap with constrained spherical deconvolution. Hum Brain Mapp 32:461–479
Hosey T, Williams G, Ansorge R (2005) Inference of multiple fiber orientations in high angular resolution diffusion imaging. Magn Reson Med 54:1480–1489
Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S (2004) Fiber tract-based atlas of human white matter anatomy. Radiology 230:77–87
Wakana S, Caprihan A, Panzenboeck MM et al (2007) Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage 36:630–644
Catani M, Allin MP, Husain M et al (2007) Symmetries in human brain language pathways correlate with verbal recall. Proc Natl Acad Sci U S A 104:17163–17168
Vernooij MW, Smits M, Wielopolski PA, Houston GC, Krestin GP, van der LA (2007) Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right- and left-handed healthy subjects: a combined fMRI and DTI study. NeuroImage 35:1064–1076
Yasmin H, Aoki S, Abe O et al (2009) Tract-specific analysis of white matter pathways in healthy subjects: a pilot study using diffusion tensor MRI. Neuroradiology 51:831–840
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. A
Overlap values for the corticospinal tract (CST) (red open circles), the arcuate fasciculus (AF) (blue open squares) and its long segment (AFl) (green open triangles) for different tract visitation thresholds (10 to 50 %, in steps of 10 %) (GIF 8 KB)
Appendix
Appendix
Fibre tractography procedure
First, whole brain tractography was performed for all individuals and imaging sessions. FA thresholds were set to 0.20 to initiate and continue tracking and the angle threshold was set to 30° for both tracts. Only fibres with a minimum length of 50 mm were considered.
Next, the CST, the AF and AFl were extracted for each individual from regions of interest (ROIs) according to a priori information of tract location [50, 51]. The tracts of interest were only delineated in the left hemisphere considering the left hemisphere lateralisation of these tracts when subjects are strongly right-handed [52, 53]. The ROI sets of all tracts of interest were manually placed only by the first author (G.K.) as a previous study reported a high interobserver reliability in tract-specific analysis [54]. This was done following the guidelines and protocols described in previous studies, where figures showing the ROI placement are also present [9, 32, 33, 51]. The CST was extracted by placing two ROIs on axial slices. The first ROI included the cerebral peduncle at the level of the decussation of the superior cerebellar peduncle. The second ROI was drawn right after the bifurcation to the motor and sensory cortex to include only primary motor cortex, and not sensory tracts [51]. The AF was extracted by placing two ROIs on coronal slices. The first ROI was selected where the AF, appearing as a green triangular shape on coronal images (indicating anterior/posterior orientation), was seen to be largest. The second ROI was selected at the level of the splenium of the corpus callosum, where the AF makes a sharp turn towards the temporal lobe [9, 32]. The AFl was defined by using the first ROI used for the delineation of the AF, and by placing a second ROI on an axial slice through which the AF passes in the superior/inferior direction [33].
The ROI sets of all tracts of interest were per individual the same for both imaging sessions. In doing so, the variability in ROI designation introduced by the operator is eliminated and does not affect the reliability results. However, we did not underestimate reliability because the protocol followed for tract delineation already reduced the variability in ROI designation to a minimum. All ROIs were placed subcortically [51] and were large enough to encompass all possible fibres belonging to the tracts of interest [33]. The subcortical placement of ROIs ensures proper delineation of tracts, such as the CST, that are known to substantially differ in volume among individuals. The large size of the ROIs ensures similar tractography results for different operators.
For each tract (corticospinal and AF) per subject and imaging session, the following parameters were calculated: average FA, average MD, average λ1, average λ┴, average length of tracts, and volume of tracts [24–26]. FA, MD, λ1 and λ┴ values were obtained by averaging across all voxels in the tract and each voxel was counted only once.
Rights and permissions
About this article
Cite this article
Kristo, G., Leemans, A., de Gelder, B. et al. Reliability of the corticospinal tract and arcuate fasciculus reconstructed with DTI-based tractography: implications for clinical practice. Eur Radiol 23, 28–36 (2013). https://doi.org/10.1007/s00330-012-2589-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-012-2589-9