Abstract
Vascular access problems lead to increased patient morbidity and mortality and place a large burden on care facilities, manpower and costs. Autogenous arteriovenous fistulas (AVF) are preferred over arteriovenous grafts (AVG) because of a lower incidence of vascular access related complications. An aggressive increase in the utilization of AVF, however, results in an increased incidence of AVF early failure and non-maturation. Increasing evidence suggests that routine preoperative assessment results in an increased utilization of functioning AVF by better selection of adequate vessels. To date, the reproducibility and standardization of assessment protocols are lacking and assessment of a single morphological parameter has not enabled adequate prediction of postoperative AVF function for individual patients. In this paper, we provide an overview of available diagnostic modalities and parameters that potentially enable better selection of adequate vessels for successful AVF creation.
Similar content being viewed by others
Introduction
End-stage renal disease (ESRD), defined as complete or near complete renal failure, is an increasingly important medical problem, ultimately requiring hemodialysis in the vast majority of patients. The incidence and prevalence of ESRD as well as the number of patients requiring hemodialysis have sharply risen over the past few years. In 2004, an estimated 1.22-million patients were on hemodialysis world-wide, representing a 20% increase in 3 years since 2001 [1, 2].
A well-functioning vascular access is the cornerstone of hemodialysis treatment and can be achieved by insertion of a central venous catheter or by surgical creation of an arteriovenous fistula or graft (Fig. 1). The access of first choice is the autogenous arteriovenous fistula (AVF) because of its better long-term performance and patency rates when compared with arteriovenous grafts (AVG) and central venous catheters (CVC). Furthermore, AVFs have lower vascular access related morbidity, mortality and healthcare costs compared with AVG and CVC [3, 4]. In contrast, prosthetic vascular grafts require about five-times more therapeutic interventions compared with AVF to keep the access functioning [5–7].
Schematic overview of a radial-cephalic arteriovenous wrist fistula (left) and a forearm arteriovenous loop graft (right). The graft anastomosed to the brachial artery and cephalic vein at the antecubital crease in an end (graft) to side (vessel) fashion. The loop has been tunneled underneath the skin to enable easy cannulation
In order to reduce long-term vascular access related complications, the Dialysis Outcome Quality Initiative (K-DOQI) and Good Nephrological Practice guidelines advocate an all AVF policy, i.e. at least 70% of all newly created accesses should consist of autogenous AVF [3, 4]. However, the major drawback of AVF creation is the relatively high frequency of early thrombosis—up to 10–20% of all newly created vascular accesses thrombose within the first week after creation—and non-maturation [8, 9]. Non-maturation is defined as an AVF being inadequate for hemodialysis due to insufficient flow-volume or insufficient venous distension after creation. Causes of non-maturation are thought to be the use of tiny vessels in addition to arterial inflow or venous outflow stenoses or occlusions [9–13]. Furthermore, the presence of large caliber side branches may also jeopardize AVF maturation due to a disadvantageous flow distribution [3, 14, 15]. Different studies have reported AVF non-maturation rates within the first months after creation from 5% up to 54% [12, 16–20].
In order to increase the number of mature and functional AVFs, adequate history taking, physical examination and preoperative assessment of upper extremity vessels is important [3, 4, 19]. Increasingly, arterial and venous diameters as well as the presence and location of preexisting atherosclerotic occlusive disease and venous stenoses, occlusions and side-branches are used to guide the choice of fistula type and location. Consequently, interest has risen in preoperative imaging of upper extremity vessels. The goal of preoperative imaging is assessment of vessel caliber and identification of sites where arteries and veins are of suboptimal quality for access purposes.
The current review will provide the radiologist with an overview of the clinical role and relative merits and shortcomings of physical examination, duplex ultrasonography (DUS), digital subtraction angiography (DSA) and contrast-enhanced magnetic resonance angiography (CE-MRA) in the preoperative work-up of patients awaiting surgical creation of a vascular access for hemodialysis.
History taking and physical examination
The cornerstone of any pre-surgical workup is a thorough history and physical examination. Negroid race, female gender, diabetes mellitus, peripheral arterial occlusive disease, previous vascular access procedures and axillary radiation therapy are associated with poor vascular access outcome [4].
Careful physical examination is important in the work-up of patients awaiting vascular access surgery. Skin lesions, local infections, generalized dermatological problems and scars, in addition to small arterial caliber and lack of pulse vigorousness as well as small venous caliber and distensibility, may indicate poor chance of successful AVF creation at standard locations and should therefore be documented and addressed [4]. However, arterial and venous assessment by physical examination can be challenging and of limited value in obese patients [12, 21]. Malovrh et al. [9] found that physical examination failed to identify suitable vessels for AVF creation in over half of all patients undergoing dialysis access surgery (n = 62/116; 54%), underscoring the need for additional information prior to access creation.
Duplex ultrasonography
DUS enables assessment of vessel patency, diameter, flow-volume and velocities. The application of DUS enables better depiction of adequate vessels for AVF creation that may not be detected by physical examination, especially in obese patients [9, 12, 21, 22]. Multiple studies have found that the application of DUS resulted in changes in surgical procedure in 31% of cases, changes in site of exploration (9.6%), a decrease in unsuccessful explorations (11% to 0%), an increase in the relative number of AVFs created (as opposed to other types of access; 64% vs 34%), and a decrease in non-maturation rates (38% to 8.3% and 66% to 46%), when compared with the use of physical examination alone [12, 16, 23–25]. However, reported cut-off values of various DUS-derived parameters (i.e. diameter, flow-volume, velocities, compliance and resistive index) are inconsistent. An aggressive approach to conform to the all AVF policy may, therefore, still result in increased early failure and non-maturation rates if based on a single DUS-derived parameter [26, 27]. It remains to be established if a combination of parameters might enable better prediction of vascular access function and minimize early failure and non-maturation rates.
Arterial assessment
Preoperative DUS examination should include assessment of the arteries from the subclavian artery down to the radial and ulnar arteries at the wrist [4]. The exact course and continuity as well as the presence of stenoses should be addressed because patients with arterial stenosis are thought to be at increased risk for developing hand and finger ischemia after AVF creation due to steal phenomena [4]. For detection of relevant stenoses (defined as >50% luminal reduction) in the upper extremity arterial system, DUS has a sensitivity and specificity of 90.9% and 100% for the subclavian artery, 93.3% and 100% for upper arm arteries, 88.6% and 98.7% for forearm arteries and for the arteries of the hand 70% and 100%, respectively [28, 29].
Another important morphological parameter apart from the presence of arterial stenosis is arterial diameter. Arteries with diameters smaller than 1.5 to 3.0 mm have been associated with increased non-maturation rates AVF [11, 24, 25, 30–33].
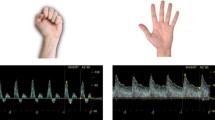
Additional parameters such as radial artery flow volume and peak systolic velocities before or during reactive hyperemia have also been reported to be predictors of AVF maturation. In Fig. 2, radial artery flow velocity changes due to fist clenching and reactive hyperemia are shown. Lockhart et al. [27], in contrast, found that arterial diameters, resistance indices and peak systolic velocities had only little if any predictive value for AVF outcome.
Duplex ultrasound assessment of radial artery flows at rest (a), during fist clenching (b) and during hyperemia after the fist clench has been released in a healthy volunteer. The arrow indicates the moment of fist clench release. Absence or diminished change in radial artery flow is associated with a higher risk of vascular access early failure and non-maturation
Venous assessment
The superficial venous system of the upper extremity is easily assessable by DUS and results in detection of more veins compared with physical examination alone [12, 21, 22]. Furthermore, it also allows for assessment of local hemodynamics, such as subclavian vein flow. A typical example of Doppler signal changes of the subclavian vein during deep inspiration in a healthy volunteer is shown in Fig. 3. The absence of changes in venous Doppler signal due to deep inspiration or loss of venous compressibility is another important finding indicative for local venous stenosis or occlusion. Preoperative detection of stenoses and obstructions is important to avoid unsuccessful surgical explorations [4]. Nack et al. [34] reported a DUS sensitivity, specificity, positive predictive value and negative predictive value of 81%, 90%, 90% and 78% respectively, for detection of venous stenosis, thrombi and occlusions when compared with DSA.
The clinical value of upper extremity DUS for detection of arterial or venous abnormalities is lower for proximal compared with distal arteries or veins, respectively. For instance, Nack et al. [34] reported progressive decreasing DUS sensitivities for detection of abnormalities in the subclavian vein (79%), innominate vein (75%), and superior vena cava (33%), when compared with DSA. This can be explained by the fact that veins course underneath bony structures such as the clavicle and ribs over a substantial length and because the central thoracic veins are relatively inaccessible by DUS because of the distance to the transducer.
As is the case for arteries, DUS-derived venous diameter is an important parameter for prediction of vascular access outcome. For assessment of venous diameter, a proximally applied cuff should be used to induce venous dilatation for better appreciation of ‘maximum’ or ‘true’ venous diameter [3, 4]. Reported venous cut-off diameters for successful AVF creation range from 1.6 to 2.6 mm [9, 19, 24, 25, 30, 33, 35]. This range may be partially explained by differences in vein mapping protocols because only few authors reported the measurement conditions and methods to achieve venous dilatation. Furthermore, DUS venous diameter measurements are observer-dependent with an inter-observer variation of 0.5 mm [36]. Recently, Planken et al. [37] have demonstrated that superficial forearm vein diameter measurements vary over time with a coefficient of variation of 27%. In addition, forearm superficial venous diameter measurement reproducibility depends on the applied venous congestion pressure and best reproducibility is achieved at venous congestion pressures >40 mmHg [38].
Preoperative contiguous length of non-diseased vein >10 cm in addition to venous diameter was predictive for the AVF function [31, 39].
Apart from venous diameter, some authors have found an association between the presence and size of venous side branches and AVF non-maturation. Wong et al. [33] suggested that a side branch <5 cm away from the planned anastomosis may impair AVF function, whereas Beathard et al. [15] have stressed the importance of the size of the venous side branches. In the aforementioned studies non-maturation was more likely in the event of a large venous side branch. Turmel-Rodrigues et al. [14], in contrast, state that venous side branches are of no importance and only come into play in the presence of a venous outflow stenosis.
Dynamic parameters to characterize upper extremity veins include flow volume and velocity measurements as well as assessment of flow wave changes due to respiratory maneuvers [4]. The capacity of superficial veins to dilate due to venous congestion (also known as compliance) has been reported to be higher within a group of patients in whom AVF creation was successful compared with patients with AVF that failed to mature (diameter increase 48% versus 11.8% at a congestion pressure of 50 mmHg) [9]. Forearm superficial venous compliance measurements, however, have been reported to be poorly reproducible due to poor reproducibility of venous diameters at low venous congestion pressures [38]. The clinical value of forearm superficial venous compliance measurements is therefore considered of little if any use.
Digital subtraction angiography
Digital subtraction angiography (DSA), using X-ray techniques and iodinated contrast-media is considered the standard of reference for assessment of upper extremity arteries and veins. A drawback, however, is that acquired data are two-dimensional (2D) projections and analysis is limited by the number and quality of the acquired projections. Furthermore, the clinical applicability in the work-up of ESRD patients is limited due to the use of iodinated nephrotoxic contrast-media that may lead to temporary or permanent deterioration of residual renal function in up to 20% of the cases [40]. Further deterioration of renal function should be avoided because loss of residual renal function is associated with higher morbidity and mortality rates [41, 42]. Another important reason to avoid the use of iodinated contrast media is the chance of renal function recovery, even after initiation of hemodialysis therapy. In addition, residual kidney functions such as secretion of organic acids and various endocrine functions cannot be provided by dialysis and should therefore be preserved as long as possible [41]. An additional obvious drawback of DSA is the invasive nature of the examination, especially for the depiction of the arterial system.
Geoffroy et al. [40] reported on the safety and clinical performance of IA-DSA using gadolinium chelates in ESRD patients and concluded that they were well tolerated with minimal impairment of renal function (mean 3%, <12% in all cases). However, others reported serious complications associated with the off-label use of available gadolinium chelates at doses exceeding 0.3 mmol/kg [43–46]. The high osmolality of contrast media is a pathogenetic factor in contrast induced nephropathy (CIN) that potentially leads to further renal function impairment. CIN is more likely in patients with pre-existing impaired renal function [43, 44, 46, 47]. Gadolinium chelates are hypertonic and the osmolality of commercially available gadolinium chelates is two- to seven-times that of plasma [43, 46]. To prevent CIN due to hypertonicity the dose should not exceed 0.3 mmol/kg. Older patients and patients with lower baseline creatinine clearance, diabetic nephropathy and low haemoglobin and albumin levels are at increased risk for developing gadolinium induced CIN, with a reported incidence between 0 and 11% [40, 47].
Another alternative to iodinated contrast-media and gadolinium chelates is carbon dioxide (CO2) gas injection [40]. In a recent report Heye et. al. [48] demonstrated that CO2 venography is an acceptable alternative (sensitivity, 97%; specificity, 85%; accuracy, 95%) for assessment of upper-extremity and central veins in patients with contraindications to conventional venography with iodinated contrast material. In Fig. 4, a CO2 venogram and corresponding conventional venogram are shown. However, CO2 injections may cause pain and the CO2 contrast technique can lead to stenosis grade overestimation [40]. The use of CO2 contrast may furthermore lead to serious complications, such as brain gas embolism, pulmonary embolism or acute cardiac arrest [40].
Arterial assessment
Upper extremity arteriography is traditionally performed by intra-arterial injection of contrast-media. Arterial access can be achieved by femoral or brachial puncture. In patients with ESRD the brachial artery approach is generally avoided because it can be painful, it may jeopardize distal perfusion and thereby maturation and function of a vascular access created distal to the puncture site. Thrombosis of the brachial artery is a serious complication due to brachial punctures for catheter access in up to 7% [49–51]. Contralateral venous injection of contrast media is a less invasive alternative with fewer complications. However, in order to achieve sufficient arterial enhancement, this technique requires a high contrast dose which is unacceptable in ESRD patients due to the potential further deterioration of residual renal function.
Venous assessment
Central venous stenosis and obstruction occur frequently after central venous catheter insertion or placement of pacemaker wires, however, often patients remain asymptomatic. Imaging of the subclavian—innominate—and superior caval veins prior to vascular access creation is important because 40% of patients with a history of central venous catheters have central venous stenosis or obstruction [52]. Examples of central venous obstructions due to pacemaker wires and central venous catheter use are shown in Figs. 5 and 6.
Venography by cannulation of an ipsilateral dorsal hand vein allows imaging of the entire cephalic or basilic veins from the hand up to the confluence of the basilic and brachial veins into the subclavian vein. Although the superficial veins of the upper extremity are connected to each other at multiple levels, the puncture site will limit venous opacification to the draining vein of the puncture site only. It is important to avoid puncturing veins proximal to the distal radius in order to preserve draining veins for future access use. In our experience, the use of a proximal tourniquet inflated to 60 mmHg enables depiction of collateral veins and improves assessment of venous diameter because of dilatation. Good inter-observer correlation coefficients have been reported for assessment of venous quality prior to access creation using conventional X-ray venography techniques and gadolinium chelates as contrast media (kappa values, cephalic vein: forearm 0.65, arm 0.88; basilic vein: forearm 0.72; arm 0.64). Opacification of upper extremity veins is generally adequate although opacification of central veins can remain problematic, even after administration of increased contrast volumes [40].
Magnetic resonance angiography (MRA)
Ongoing technical improvements have made MRA an important and valuable imaging modality in recent years in the preoperative workup of patients with ESRD. With the introduction of contrast-enhanced MRA techniques, the role of non-enhanced time-of-flight (TOF) MRA has declined as it is prone to artifacts and stenoses are frequently overestimated [53]. Because of this reason, we only discuss contrast-enhanced MRA (CE-MRA).
CE-MRA protocols enable image acquisition with high spatial (sub-millimeter voxel-size) and temporal resolutions (<20 s per dynamic scan) with good to excellent image quality [54, 55]. For arterial imaging 0.2–0.3 mmol/kg 0.5 M extracellular gadolinium chelate contrast medium is injected in a contralateral antecubital or dorsal hand vein. For venous imaging diluted (1:15) contrast media is injected in an ipsilateral dorsal hand vein.
Because intra-thoracic vessels are prone to movement during respiration, patients should hold their breath for about 15–20 s depending on scan protocol and technical factors related to system performance. Contrast medium is injected at speeds up to 3.0 ml/s, followed by 25 ml of saline flush. Spatial resolution in recent reports is typically in the order of 1.0×1.0×1.2 mm3 (cranio-caudal/frequency direction×left-right/phase-encoding directions×antero-posterior/slice direction) [54]. Using this approach for imaging, upper extremity arteries and veins can be visualized with high accuracy [54]. Because the average upper extremity length of an adult is about 70–80 cm, depicting the entire upper extremity requires imaging of at least two fields-of-view because of the limited MR-bore length. An example of a preoperative CE-MR arteriogram and venogram of an ESRD-patient is shown in Fig. 7.
Example of selective CE-MRA of upper extremity arteries (a) and veins (b), by contralateral and ipsipateral injection of contrast media, respectively. Images of the entire upper extremity vasculature were acquired in four consecutive phases. First distal arteries (forearm), second proximal arteries (upper arm and thorax), third proximal veins (upper arm and thorax) and fourth distal veins (forearm). (sa subclavian artery, ba brachial artery, ra radial artery, ua ulnar artery, sv subclavian vein, cv cephalic vein, bv basilic vein)
Synchronization of peak arterial or venous contrast concentration with sampling of central k-space profiles is very important in order to obtain selective imaging of arteries or veins, respectively [56]. This is typically done by performing a timing sequence with 1–2 ml contrast medium prior to the contrast-enhanced acquisition, or by the use of real-time bolus monitoring software. In order to improve both spatial and temporal resolution multi-element surface coils can be used to enable parallel imaging techniques [57]. Apart from their parallel imaging capabilities these coils greatly improve vessel-to-background contrast.
Arterial assessment
Planken et al. [54] reported a multi-phase approach using multiple dynamic scans that resulted in good to excellent subjective image quality images of upper extremity arteries down to the wrist. Assessment of the arterial palmar arch and digital arteries remains cumbersome, however, with the currently acquired spatial resolutions, except when using timed arterial compression [55]. An important advance in this regard is the recent clinical introduction of blood pool contrast media which enables ultra-high spatial resolution scans for assessment of the palmar arch and digital arteries (Fig. 8) [58].
Ultra high resolution (acquired voxel size: 0.4×0.4×0.4 mm3) steady state CEMRA of the arterial palmar arch and the proximal digital arteries, 45 s after injection of a blood pool agent. Although, both arteries and veins are equally opacified during steady state and separation of arteries and veins can be difficult, the arterial palmar arch (arrowheads) can be identified as well as accompanying veins (arrow)
Although CE-MRA is believed to be highly accurate for detection of arterial stenoses and obstructions there are no studies about the accuracy and reproducibility for detection of thoracic and upper extremity arterial stenoses in ESRD patients. A typical example of a subclavian artery stenosis depicted by both CE-MRA and DSA is shown in Fig. 9.
Venous assessment
Reported CE-MR venography techniques use either direct injection of diluted contrast-media (1:15 to 1:25 ml of gadolinium chelate in saline solution) in the ipsilateral extremity or contralateral intravenous injection of non-diluted contrast-media, and acquisition during delayed venous enhancement after initial arterial first pass [59, 60]. Both techniques have their strengths and weaknesses. Direct venography with application of a proximal blood pressure cuff inflated to 60 mmHg yields better vessel opacification with lower contrast dose compared with the contralateral injection approach. Examples of a central venous stenosis and obstruction by direct MR venography are shown in Figs. 10 and 11.
Contrast-enhanced MR venography using delayed venous enhancement techniques showed relatively poor performance for detection of central venous stenosis and occlusions (sensitivity and specificity 50% and 80%) [61, 62]. Direct CE-MR venography is easy to perform, well tolerated and highly accurate for detection of venous stenosis and obstructions in the upper extremity and central veins [59, 60, 63–65]. Furthermore, direct CE-MR venography diameter measurements are more accurate compared with duplex ultrasonography when using surgical measurements as standard of reference [54]. Because of the lower contrast dose, better vessel opacification and accuracy, direct CE-MR venography seems to be the method of first choice.
Although CE-MRA is a promising and attractive modality for imaging the upper extremity arteries and veins, there are only sparse data about the clinical value in the work-up prior to vascular access creation. Future studies are needed to determine the clinical significance of adding CE-MRA to the preoperative work up prior to vascular access creation.
Safety of gadolinium containing contrast agents in patients with ESRD
The most widely used gadolinium chelates for CE-MRA purposes are gadopentate dimeglumine (Magnevist; Schering, Berlin, Germany), gadoterate dimeglumine (Dotarem; Guerbet, Aulnay, France), gadodiamide (Omniscan; GE Health, Oslo, Norway), gadoversetamide (OptiMARK; Mallinckrodt, St Louis, Mo.) and gadoteridol (ProHance; Bracco Diagnostics, Milan, Italy). The total incidence of adverse events related to gadolinium use for CE-MRA appears to be less than 5%. The incidence of any single adverse event is approximately 1%, or lower. By far the most common events are nausea, headache, and emesis [43]. When used intravenously, no detectable nephrotoxicity has been reported and the rates of adverse events are extremely low at the doses used [66–68].
Recently, however, concerns have arisen regarding the accumulation of free gadolinium in patients with renal failure [43]. During the last decade approximately 200 cases of nephrogenic systemic fibrosis (NSF), previously known as nephrogenic fibrosing dermopathy, have been reported worldwide [69, 70]. The reported clinical sings and symptoms of NSF are subacute progressive swelling of extremities followed by more proximal involvement and severe skin induration, pain, muscle restlessness and loss of skin flexibility. NSF can lead to serious physical disability and wheelchair requirement [70]. The incidence of NSF is low and the pathophysiology is unknown [70]. To date, all gadolinium chelates and gadodiamide (Omniscan) in particular, are believed to be associated with NSF [71, 72]. Because of these reported adverse events and the suspected causative relationship, the use of gadolinium-containing contrast agents should be discouraged in ESRD patients until the causative relationship is proven untrue [71, 72].
Summary and conclusions
Although history taking and physical examination are valuable in the pre-operative work-up of ESRD-patients prior to hemodialysis access creation, according to current guidelines, additional diagnostic modalities are indicated to assess arterial and venous vessel quality, or in case of suspected central venous obstruction.
The ultimate goal of preoperative assessment is to prevent non-maturation by optimal selection of the site of anastomosis and by identification and treatment of preexisting lesions before vascular access creation. However, even with the use of available diagnostic tools, non-maturation rates remain unacceptably high and standardized protocols for preoperative DUS, DSA or CE-MRA are lacking. Reported cut-off values for studied parameters are inconsistent, which might be explained by differences between research groups in measurement protocols and differences in the parameters measured.
The phenomenon of non-maturation remains poorly understood. To date, assessment of a single morphological or functional parameter has not enabled adequate prediction of postoperative AVF function for individual patients. To further unravel causes of AVF non-maturation more research is necessary. Furthermore, determination of measurement reproducibility for each parameter and each diagnostic modality is necessary before a standardized preoperative diagnostic approach can be implemented.
References
Grassmann A, Gioberge S, Moeller S, Brown G (2005) ESRD patients in 2004: global overview of patient numbers, treatment modalities and associated trends. Nephrol Dial Transplant 20: 2587–2593
Moeller S, Gioberge S, Brown G (2002) ESRD patients in 2001: global overview of patients, treatment modalities and development trends. Nephrol Dial Transplant 17:2071–2076
Vascular Access 2006 Work Group (2006) Clinical practice guidelines for vascular access. Am J Kidney Dis 48(Suppl 1):S176–S247
Tordoir JH, Mickley V (2003) European guidelines for vascular access: clinical algorithms on vascular access for haemodialysis. Edtna Erca J 29:131–136
Staramos DN, Lazarides MK, Tzilalis VD, Ekonomou CS, Simopoulos CE, Dayantas JN (2000) Patency of autologous and prosthetic arteriovenous fistulas in elderly patients. Eur J Surg 166:777–781
Kherlakian GM, Roedersheimer LR, Arbaugh JJ, Newmark KJ, King LR (1986) Comparison of autogenous fistula versus expanded polytetrafluoroethylene graft fistula for angioaccess in hemodialysis. Am J Surg 152:238–243
Leapman SB, Boyle M, Pescovitz MD, Milgrom ML, Jindal RM, Filo RS (1996) The arteriovenous fistula for hemodialysis access: gold standard or archaic relic? Am Surg 62:652–656; discussion 656–657
Rooijens PP, Tordoir JH, Stijnen T, Burgmans JP, Smet de AA, Yo TI (2004) Radiocephalic wrist arteriovenous fistula for hemodialysis: meta-analysis indicates a high primary failure rate. Eur J Vasc Endovasc Surg 28:583–589
Malovrh M (2002) Native arteriovenous fistula: preoperative evaluation. Am J Kidney Dis 39:1218–1225
Konner K, Hulbert-Shearon TE, Roys EC, Port FK (2002) Tailoring the initial vascular access for dialysis patients. Kidney Int 62:329–338
Malovrh M (1998) Non-invasive evaluation of vessels by duplex sonography prior to construction of arteriovenous fistulas for haemodialysis. Nephrol Dial Transplant 13:125–129
Mihmanli I, Besirli K, Kurugoglu S, Atakir K, Haider S, Ogut G, Numan F, Canturk E, Sayin AG (2001) Cephalic vein and hemodialysis fistula: surgeon's observation versus color Doppler ultrasonographic findings. J Ultrasound Med 20:217–222
Zeebregts C, van den Dungen J, Bolt A, Franssen C, Verhoeven E, van Schilfgaarde R (2002) Factors predictive of failure of Brescia-Cimino arteriovenous fistulas. Eur J Surg 168:29–36
Turmel-Rodrigues L, Mouton A, Birmele B, Billaux L, Ammar N, Grezard O, Hauss S, Pengloan J (2001) Salvage of immature forearm fistulas for haemodialysis by interventional radiology. Nephrol Dial Transplant 16:2365–2371
Beathard GA, Arnold P, Jackson J, Litchfield T (2003) Aggressive treatment of early fistula failure. Kidney Int 64:1487–1494
Allon M, Robbin ML (2002) Increasing arteriovenous fistulas in hemodialysis patients: problems and solutions. Kidney Int 62:1109–1124
Miller PE, Tolwani A, Luscy CP, Deierhoi MH, Bailey R, Redden DT, Allon M (1999) Predictors of adequacy of arteriovenous fistulas in hemodialysis patients. Kidney Int 56:275–280
Patel ST, Hughes J, Mills JL Sr (2003) Failure of arteriovenous fistula maturation: an unintended consequence of exceeding dialysis outcome quality Initiative guidelines for hemodialysis access. J Vasc Surg 38:439–445; discussion 445
Ascher E, Gade P, Hingorani A, Mazzariol F, Gunduz Y, Fodera M, Yorkovich W (2000) Changes in the practice of angioaccess surgery: impact of dialysis outcome and quality initiative recommendations. J Vasc Surg 31:84–92
Tordoir JH, Rooyens P, Dammers R, van der Sande FM, de Haan M, Yo TI (2003) Prospective evaluation of failure modes in autogenous radiocephalic wrist access for haemodialysis. Nephrol Dial Transplant 18:378–383
Vassalotti JA, Falk A, Cohl ED, Uribarri J, Teodorescu V (2002) Obese and non-obese hemodialysis patients have a similar prevalence of functioning arteriovenous fistula using pre-operative vein mapping. Clin Nephrol 58:211–214
Katz ML, Comerota AJ, DeRojas J, Bowman G, Czeredarczuk M, White JV (1987) B-mode imaging to determine the suitability of arm veins for primary arteriovenous fistulae. J Vasc Technol 11:172–174
Allon M, Bailey R, Ballard R, Deierhoi MH, Hamrick K, Oser R, Rhynes VK, Robbin ML, Saddekni S, Zeigler ST (1998) A multidisciplinary approach to hemodialysis access: prospective evaluation. Kidney Int 53:473–479
Robbin ML, Gallichio MH, Deierhoi MH, Young CJ, Weber TM, Allon M (2000) US vascular mapping before hemodialysis access placement. Radiology 217:83–88
Silva MB Jr, Hobson RW 2nd, Pappas PJ, Jamil Z, Araki CT, Goldberg MC, Gwertzman G, Padberg FT Jr (1998) A strategy for increasing use of autogenous hemodialysis access procedures: impact of preoperative noninvasive evaluation. J Vasc Surg 27:302–307; discussion 307–308
Robbin ML, Chamberlain NE, Lockhart ME, Gallichio MH, Young CJ, Deierhoi MH, Allon M (2002) Hemodialysis arteriovenous fistula maturity: US evaluation. Radiology 225:59–64
Lockhart ME, Robbin ML, Allon M (2004) Preoperative sonographic radial artery evaluation and correlation with subsequent radiocephalic fistula outcome. J Ultrasound Med 23:161–168; quiz 169–171
Wittenberg G, Landwehr P, Moll R, Tschammler A, Buschmann B, Krahe T (1993) [Interobserver variability of dialysis shunt flow measurements using color coated duplex sonography]. Rofo 159:375–378
Wittenberg G, Schindler T, Tschammler A, Kenn W, Hahn D (1998) [Value of color-coded duplex ultrasound in evaluating arm blood vessels—arteries and hemodialysis shunts]. Ultraschall Med 19:22–27
Mendes RR, Farber MA, Marston WA, Dinwiddie LC, Keagy BA, Burnham SJ (2002) Prediction of wrist arteriovenous fistula maturation with preoperative vein mapping with ultrasonography. J Vasc Surg 36:460–463
Lemson MS, Leunissen KM, Tordoir JH (1998) Does pre-operative duplex examination improve patency rates of Brescia-Cimino fistulas? Nephrol Dial Transplant 13:1360–1361
Leblanc M, Saint-Sauveur E, Pichette V (2003) Native arterio-venous fistula for hemodialysis: What to expect early after creation? J Vasc Access 4:39–44
Wong V, Ward R, Taylor J, Selvakumar S, How TV, Bakran A (1996) Factors associated with early failure of arteriovenous fistulae for haemodialysis access. Eur J Vasc Endovasc Surg 12:207–213
Nack TL, Needleman L (1992) Comparison of duplex ultrasound and contrast venography for evaluation of upper extremity venous disease. J Vasc Technol 16:69–73
Brimble KS, Rabbat ChG, Treleaven DJ, Ingram AJ (2002) Utility of ultrasonographic venous assessment prior to forearm arteriovenous fistula creation. Clin Nephrol 58:122–127
Lees TA, Manzo R, Strandness DE, Appleton D (1994) Observer variation in the measurement of venous diameters using duplex scanning. J Vasc Technol 18:177–180
Planken RN, Keuter XH, Hoeks AP, Kooman JP, van der Sande FM, Kessels AG, Leiner T, Tordoir JH (2006) Diameter measurements of the forearm cephalic vein prior to vascular access creation in end-stage renal disease patients: graduated pressure cuff versus tourniquet vessel dilatation. Nephrol Dial Transplant 21:802–806
Planken RN, Keuter XH, Kessels AG, Hoeks AP, Leiner T, Tordoir JH (2006) Forearm cephalic vein cross-sectional area changes at incremental congestion pressures: towards a standardized and reproducible vein mapping protocol. J Vasc Surg 44:353–358
Allon M, Lockhart ME, Lilly RZ, Gallichio MH, Young CJ, Barker J, Deierhoi MH, Robbin ML (2001) Effect of preoperative sonographic mapping on vascular access outcomes in hemodialysis patients. Kidney Int 60:2013–2020
Geoffroy O, Tassart M, Le Blanche AF, Khalil A, Duedal V, Rossert J, Bigot J M, Boudghene FP (2001) Upper extremity digital subtraction venography with gadoterate meglumine before fistula creation for hemodialysis. Kidney Int 59:1491–1497
Jansen MA, Hart AA, Korevaar JC, Dekker FW, Boeschoten EW, Krediet RT (2002) Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int 62:1046–1053
Merkus MP, Jager KJ, Dekker FW, de Haan RJ, Boeschoten EW, Krediet RT (2000) Predictors of poor outcome in chronic dialysis patients: The Netherlands Cooperative Study on the Adequacy of Dialysis. The NECOSAD study group. Am J Kidney Dis 35:69–79
Shellock FG, Kanal E (1999) Safety of magnetic resonance imaging contrast agents. J Magn Reson Imaging 10:477–484
Sam AD 2nd, Morasch MD, Collins J, Song G, Chen R, Pereles FS (2003) Safety of gadolinium contrast angiography in patients with chronic renal insufficiency. J Vasc Surg 38:313–318
Nyman U, Elmstahl B, Leander P, Nilsson M, Golman K, Almen T (2002) Are gadolinium-based contrast media really safer than iodinated media for digital subtraction angiography in patients with azotemia? Radiology 223:311–318; discussion 328–319
Nyman U, Golman K (2004) Regarding “Safety of gadolinium contrast angiography in patients with chronic renal insufficiency”. J Vasc Surg 40:204; author reply 204
Ergun I, Keven K, Uruc I, Ekmekci Y, Canbakan B, Erden I, Karatan O (2006) The safety of gadolinium in patients with stage 3 and 4 renal failure. Nephrol Dial Transplant 21:697–700
Heye S, Maleux G, Marchal GJ (2006) Upper-extremity venography: CO2 versus iodinated contrast material. Radiology 241:291–297
Heenan SD, Grubnic S, Buckenham TM, Belli AM (1996) Transbrachial arteriography: indications and complications. Clin Radiol 51:205–209
Grollman JH Jr, Marcus R (1988) Transbrachial arteriography: techniques and complications. Cardiovasc Intervent Radiol 11:32–35
Watkinson AF, Hartnell GG (1991) Complications of direct brachial artery puncture for arteriography: a comparison of techniques. Clin Radiol 44:189–191
Surratt RS, Picus D, Hicks ME, Darcy MD, Kleinhoffer M, Jendrisak M (1991) The importance of preoperative evaluation of the subclavian vein in dialysis access planning. AJR Am J Roentgenol 156:623–625
Meaney JF (2003) Magnetic resonance angiography of the peripheral arteries: current status. Eur Radiol 13:836–852
Planken RN, Tordoir JHM, de Haan MW, Backes WH, van Engelshoven JMA, Leiner T (2005) In: Mickley V (ed) 4th International Congress of the Vascular Access Society (VAS), vol 23. Karger, Berlin, pp 227–261
Wentz KU, Frohlich JM, von Weymarn C, Patak MA, Jenelten R, Zollikofer CL (2003) High-resolution magnetic resonance angiography of hands with timed arterial compression (tac-MRA). Lancet 361:49–50
Maki JH, Chenevert TL, Prince MR (1996) Three-dimensional contrast-enhanced MR angiography. Top Magn Reson Imaging 8:322–344
Weiger M, Pruessmann KP, Kassner A, Roditi G, Lawton T, Reid A, Boesiger P (2000) Contrast-enhanced 3D MRA using SENSE. J Magn Reson Imaging 12:671–677
Hartmann M, Wiethoff AJ, Hentrich HR, Rohrer M (2006) Initial imaging recommendations for Vasovist angiography. Eur Radiol 16(Suppl 2):B15–B23
Ruehm SG, Zimny K, Debatin JF (2001) Direct contrast-enhanced 3D MR venography. Eur Radiol 11:102–112
Shinde TS, Lee VS, Rofsky NM, Krinsky GA, Weinreb JC (1999) Three-dimensional gadolinium-enhanced MR venographic evaluation of patency of central veins in the thorax: initial experience. Radiology 213:555–560
Haire WD, Lynch TG, Lund, GB, Lieberman RP, Edney JA (1991) Limitations of magnetic resonance imaging and ultrasound-directed (duplex) scanning in the diagnosis of subclavian vein thrombosis. J Vasc Surg 13:391–397
Baarslag HJ, Van Beek EJ, Reekers JA (2004) Magnetic resonance venography in consecutive patients with suspected deep vein thrombosis of the upper extremity: initial experience. Acta Radiol 45:38–43
Li W, David V, Kaplan R, Edelman RR (1998) Three-dimensional low dose gadolinium-enhanced peripheral MR venography. J Magn Reson Imaging 8:630–633
Goyen M, Barkhausen J, Kuehl H, Goehde SC, Kroger K, Bosk S, Debatin JF, Ruehm SG (2001) [Contrast-enhanced 3D MR venography of central thoracic veins: preliminary experience]. Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr 173:356–361
Thornton MJ, Ryan R, Varghese JC, Farrell MA, Lucey B, Lee MJ (1999) A three-dimensional gadolinium-enhanced MR venography technique for imaging central veins. AJR Am J Roentgenol 173:999–1003
Cochran ST, Bomyea K, Sayre JW (2001) Trends in adverse events after IV administration of contrast media. AJR Am J Roentgenol 176:1385–1388
Prince MR, Arnoldus C, Frisoli JK (1996) Nephrotoxicity of high-dose gadolinium compared with iodinated contrast. J Magn Reson Imaging 6:162–166
Tombach B, Bremer C, Reimer P, Kisters K, Schaefer RM, Geens V, Heindel W (2001) Renal tolerance of a neutral gadolinium chelate (gadobutrol) in patients with chronic renal failure: results of a randomized study. Radiology 218:651–657
Thomsen HS (2006) Nephrogenic systemic fibrosis: a serious late adverse reaction to gadodiamide. Eur Radiol 16:2619–2621
Marckmann P, Skov L, Rossen K, Dupont A, Damholt MB, Heaf JG, Thomsen HS (2006) Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 17:2359–2362
Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE (2007) Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology 242:647–649
Sadowski EA, Bennett LK, Chan MR, Wentland AL, Garrett AL, Garrett RW, Djamali A (2007) Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology 243:148–157
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 2.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by-nc/2.0/.
About this article
Cite this article
Planken, R.N., Tordoir, J.H.M., Duijm, L.E.M. et al. Current techniques for assessment of upper extremity vasculature prior to hemodialysis vascular access creation. Eur Radiol 17, 3001–3011 (2007). https://doi.org/10.1007/s00330-007-0662-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-007-0662-6