Abstract
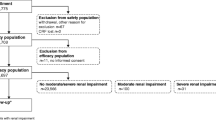
The purpose of this study was to review the rate of adverse events after contrast medium administration in the general population and at-risk patients (renal impairment, heart failure (NYHA III or IV), hypotension or hypertension, coronary artery disease, previous reaction to contrast media, asthma and/or allergies, dehydration, diabetes mellitus, poor general condition) under daily practice conditions in a post-marketing surveillance study. Two hundred and ten radiologists conducted various X-ray examinations in 52,057 patients. To document the safety of iobitridol in routine use, all patients undergoing X-ray examinations were included. Exclusion criteria were contraindications listed in the locally approved summary of product characteristics. The adverse event rate was 0.96% (at-risk patients 1.39%); the rate of serious adverse events 0.044% in all patients (at-risk patients 0.057%). Adverse events occurred more often in women than in men (P<0.001). In patients who had previously reacted to a contrast medium, adverse events were reported in 3.43% with mild to moderate symptoms. In 47.76% of these patients, a premedication was administered. There was no difference in the frequency of adverse events and serious adverse events whether premedicated or not (P=0.311 and P=0.295, respectively). Iobitridol was well-tolerated in 99.04% of cases (at-risk patients 98.61%).
Similar content being viewed by others
References
Petersein J, Peters CR, Wolf M, Hamm B (2003) Results of the safety and efficacy of iobitridol in more than 61,000 patients. Eur Radiol 13:2006–2011
Katayama H, Yamaguchi K, Kozuka T, Takashima T, Seez P, Matsuura K (1990) Adverse reactions to ionic and nonionic contrast media. Radiology 175:621–628
Schrott VKM, Behrends B, Clauss W et al (1986) Iohexol in der Ausscheidungsurographie. Ergebnisse des Drug-monitoring. Fortschr Med 104 153–156
Bettman MA, Heeren T, Greenfield A et al (1997) Adverse events with radiographic contrast agents: results of the SCVIR contrast agent registry. Radiology 203:611–620
Palmer FJ (1988) The RACR survey of intravenous contrast media reactions final report. Australas Radiol 32:426–428
Mortele KJ, Oliva MR, Ondategui S, Ros PR, Silverman SG (2005) Universal use of nonionic iodinated contrast medium for CT: evaluation of safety in a large urban teaching hospital. AJR Am J Roentgenol. 2005 Jan 184:31–34
Lasser EC, Berry CC, Mishkin MM et al (1994) Pretreatment with corticosteroids to prevent adverse reactions to non-ionic contrast media. AJR Am J Roentgenol 162:523–526
Lang DM, Alpern MB, Visintainer PF, Smith ST (1995) Gender risk for anaphylactoid reaction to radiographic contrast media. J Allergy Clin Immunol 95:813–817
Lasser EC, Lyon SG, Berry CC (1997) Reports on contrast media reactions: analysis of data from reports to the U.S. Food and Drug Administration. Radiology 203:605–610
Spring DB, Bettmann MA, Barkan HE (1997) Deaths related to iodinated contrast media reported spontaneously to the U.S. Food and Drug Administration, 1979–1994: effect of the availability of low-osmolality contrast media. Radiology 204:333–337
Steinberg EP, Moore RD, Powe NR et al (1992) Safety and cost effectiveness of high- osmolality as compared with low-osmolality contrast material in patients undergoing cardiac angiography. N Engl J Med 326:425
Wagner HJ, Evers JP, Hoppe M, Klose KJ (1997) Must the patient fast before intravascular injection of a non-ionic contrast medium? Results of a controlled study. Fortschr Röntgenstr 166:370–375
Eloy R, Corot C, Belleville J (1991) Contrast media for angiography. Physicochemical properties, pharmacokinetics and biocompatibility. Clin Mat 7:89–197
Sickmüller B, Müller J. (2004) Observational studies - The German experience. Regulatory Affairs Journals RAJ Pharma, Apr 2004
WHO (1998) International monitoring of adverse reactions to drugs: adverse reaction terminology. WHO Collaborating Centre for International Drug Monitoring, Uppsala
Idée JM, Pinès E, Prigent P, Corot C (2005) Allergy-like reactions to iodinated contrast agents. A critical analysis. Fundam Clin Pharmacol 19:263–281
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vogl, T.J., Honold, E., Wolf, M. et al. Safety of iobitridol in the general population and at-risk patients. Eur Radiol 16, 1288–1297 (2006). https://doi.org/10.1007/s00330-005-0061-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-005-0061-9