Abstract
Waders that breed in the sub-Arctic are one of the groups most threatened by climate change. At the same time, wader breeding success also can vary as a function of fluctuations in the numbers of predators and rodents (an alternative prey for the predators). How climate change could influence these foodweb interactions remains poorly studied. In this study, we analysed the effects of ecological (e.g. vole/lemming and predator abundance) and environmental factors (e.g. snow cover) on the breeding success of waders in sub-Arctic Lapland. We monitored more than 500 wader nests during six breeding seasons, which spanned a full rodent cycle and one year of exceptionally late snow melt. Nest predation rate, and thus wader breeding success, did not vary as a function of predator or rodent abundance. However, predation rate was exceptionally high in the year with a late snow melt. More variability in climate is expected for the future, where more precipitation and cold spring temperatures resulting in late snow melt will be more frequent, influencing the rodent and predator numbers, and therefore wader breeding success in the sub-Arctic. Snow would limit the number of open areas for nesting and hence predators would then be able to find these nests more easily. Additionally, predators might concentrate their efforts on alternative prey if snow has reduced their capacity to find other food sources. And, ultimately, changes in the rodent fluctuations could affect the final outcome of predators.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Effects of climate change on ecosystems have been studied extensively and throughout the world (Walther et al. 2002; Rosenzweig et al. 2007; Walther 2010). However, climate change scenarios indicate that global warming is expected to be most pronounced, both in absolute and relative terms, in the Arctic and sub-Arctic regions (IPCC 2014). In addition to temperature, changes in precipitation are also predicted (Callaghan et al. 2011; IPCC 2014). This might be especially true for Arctic and sub-Arctic ecosystems where an increase in precipitation means an increase in snow depth and snow cover (Popova 2004; Serreze et al. 2007). Effects of an increase in temperature could be offset by effects of an increase in precipitation (snow), and one possible outcome of climate change could be a shorter snow-free period during the summer (Radionov et al. 2004).
A long-term increase in snow depth has been observed for Arctic and sub-Arctic regions (Callaghan et al 2011). These predictions and observations are supported by statements by Sámi reindeer herders from northern Sweden (Callaghan et al. 2010; Riseth et al. 2010). They, for example, have stated that “terrain elements that determined animal movements in the summer are now snow covered: reindeer now find new passes and roam over a wider area”, “snow-covered areas and snow patches persist longer into the summer in high mountain areas” (Riseth et al. 2010).
To understand how climate change will affect Arctic and sub-Arctic ecosystems, it is thus essential to study the effects not only of an increase in snow cover but also of an increase in temperature. Waders are a prominent and characteristic species group of Arctic and sub-Arctic ecosystems, and could be considered sentinels of changing ecosystems (Meltofte et al. 2007; Van Gils et al. 2016). Waders are expected to be sensitive to variation in snow cover as they have a limited time window for reproduction at higher latitudes. A shorter breeding season in connection with an increase in winter snowfall could be limiting for wader populations as food availability could mismatch with hatching timing. It is therefore important to establish the relationships between spring snow cover and key annual cycle events such as the timing of the onset of egg laying.
In addition, wader nesting success is highly dependent on rates of predation (MacDonald and Bolton 2008). For waders breeding in the Arctic, nesting success is shaped by regular fluctuations in the number of voles/lemmings and predators (Angelstam et al. 1984; Meltofte et al. 2007; McKinnon et al. 2014). Many predator species take advantage of the cyclic small mammal populations, e.g. Arctic fox Vulpes lagopus), red fox Vulpes vulpes, rough-legged buzzard Buteo lagopus and mustelids. These predators can also switch from feeding on voles and lemmings when these are not abundant to depredate species such as waders, “the alternative prey hypothesis” (Roselaar 1979; Summers 1986; Underhill et al. 1993; Ebbinge and Spaans 2002; Quakenbush et al. 2004; Perkins et al. 2007). However, little is known about how predator–prey multi-specific relationships are affected by a variation in spring snow cover (Gilg et al. 2009).
In this study, we describe the nesting success of waders breeding in sub-Arctic Lapland in 2008–2013, in relation to variation in ecological conditions (e.g. vole/lemming and predator abundance) and environmental conditions (e.g. snow cover). We describe how the timing of nesting (onset of incubation) and predator–prey relationships are affected by snow cover, providing new insights into how wader nesting success could change under climate change scenarios.
Material and methods
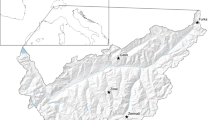
Fieldwork was conducted in the Vindelfjällen Nature Reserve (65°57′N and 16°12′E) (Fig. 1) during breeding seasons of 2008–2013. The area is a Special Protected Area (SPA) for birds under the European Commission Birds Directive (Natura 2000), as well as a designated Important Bird Area (IBA) (BirdLife International 2017). Most of the reserve lies within the alpine zone. The area is treeless above 800 m in altitude and characterized by tundra habitats such as dry heath, grass heath, dry fen and rocks (Staafjord 2012). Within the nature reserve, we covered three different subareas (Björkfjället, Gelmetje and Raurejaure) situated near the village Ammarnäs. The total study area measures about 60 km2.
Daily temperature data and information on snow depth were obtained from the nearest weather station in Boksjö, situated 36 km south of the study area, at 470 m a.s.l. (Swedish Meteorological and Hydrological Institute, SMHI). Snow depth is measured every morning from the weather stations and reported automatically to SMHI. In 2012, nearly the entire study area was covered by snow when we arrived in the first week of June. Therefore, in 2012, we estimated snow cover for the three subareas at each visit by observing the area from high points on top of the hills. In the other years, snow cover was not estimated by us, since the snow had melted before our arrival.
In the area, the main rodent species are the Norwegian lemming Lemmus lemmus and the field vole Microtus agrestis. Their numbers fluctuate in cycles of about 3 to 5 years (Angerbjörn et al. 2001). Data on the abundance of voles and lemmings were obtained from the project “Environmental monitoring of rodents” from the Swedish University of Agricultural Sciences (www.slu.se/mo-smagnagare). Data are collected using a density index based on two captures per year: one in spring and one in autumn. In Ammarnäs, a total of 2200 traps are used annually, at a density of 50 traps per ha, and the total area covered the trap line consists of 40 ha.
Red fox and stoat Mustela erminea are the main mammalian predators of wader nests present in the study area (pers. obs.), and as such are probably responsible for the great majority of predation events. Avian predators include long-tailed skua Stercorarius longicaudus and common raven Corvus corax. In a pilot study in 2011, when a number of automated trail cameras were placed near Eurasian golden plover Pluvialis apricaria nests, two nests were depredated by long-tailed skuas, one by a common raven and one by a red fox. In addition, the cameras registered four predation events by reindeer Rangifer tarandus. The latter occurred mainly when reindeer herds gathered in high densities in the east of the Raurejaure area.
Data on the relative abundance of mammalian predators were obtained from the Wildlife Triangle Scheme (Stoessel et al. 2017). This scheme consists of snowtracking surveys that are conducted in March and April following Lindén et al. (1996). The numbers of tracks of predators were surveyed along triangular-shaped transects, with a total length of 12 km (4 km per side of the triangle). Triangles were situated in treeless tundra throughout the nature reserve. The exact same triangles were surveyed throughout the study period. Surveys were performed in good snow and weather conditions from a snowmobile. In order to ensure only fresh tracks were counted, surveys were timed the day after old tracks were erased by snowfall or wind (Lindén et al. 1996). For each track, the species was identified and the exact location of the track was recorded using a handheld GPS device. A track index was calculated for each year, as the mean number of recorded tracks of predators per triangle per year. For this study, we only used the data for three triangles situated near the study area. Data on the abundance of Long-tailed Skuas were obtained from the LUVRE monitoring scheme using territory mapping (www.luvre.org). Additionally, Long-tailed Skua nests were recorded every year by spotting the nest when the adult was incubating.
Wader nests were situated in areas holding high densities of breeding waders according to the long-term LUVRE project (www.luvre.org). These areas host nests of the following species: dunlin Calidris alpina, Eurasian golden plover, red-necked phalarope Phalaropus lobatus and common redshank Tringa totanus. Other species present in the area were Temminck’s Stint Calidris temminckii, ruff Calidris pugnax, common ringed plover Charadrius hiaticula and Eurasian dotterel Charadrius morinellus. Nests were situated by following adults back to the nest or by “rope-dragging” (Labisky 1957). For completed clutches, hatching dates were determined by the egg flotation method (Liebezeit et al. 2007; Hansen et al. 2011). For incomplete clutches (i.e. found during the laying period), the hatching date was simply determined by adding the length of the incubation period to the date when 4 eggs were presumed to have been laid, assuming 1 egg was laid per day (20 days for red-necked phalarope, 21 days for dunlin and Temminck's Stint, 22 days for Ruff, 24 days for common redshank, 28 days for Eurasian golden plover, cf. Harrison and Castell 2004). Start of egg laying was then calculated back from the hatching date and resting one day extra for each egg present in the nest.
Nests were checked every 2–7 days (mean = 3.66 days) until hatching or until the nest was depredated or abandoned. Nests were considered to be still active when the eggs were warm, but considered abandoned when the eggs were cold during two consecutive visits. Nests were considered to have been depredated when eggs had disappeared before the estimated hatching date. Clutches were considered to have been successfully hatched when the chicks were found in or close to the nest, or when there were clear signs of hatching (small egg fragments in nest, egg cap near the nest).
Daily survival rates (DSR) were calculated using Shaffer logistic exposure analyses (Shaffer 2004), where the exposure interval (in days) for each nest begins on the day the nest was found and ends on the day eggs hatched or the clutch was destroyed or abandoned or was no longer under observation. When the day of destruction or abandonment is unknown, it was assumed to have occurred midway between the last two visits. For nests observed on 2 or more days but not revisited to determine their fate, the day of the last visit marked the end of the exposure interval. For a further study of the biotic and abiotic factors, a general linear model was implemented using a logistic exposure function defined by Rotella et al. (2004). R software (R Development Core Team 2008) was used to perform the model where the factors were day of the season, snow depth during the preceding May, abundance of Long-tailed Skuas, predator index and rodent index lagged one year. Rodent numbers are lagging one year since the effect on waders is maximum the year after. Rodent numbers determine the productivity of predators during the summer and their survival in winter. This is therefore determining the abundance of predators the spring after when waders come back to breed in the area. The response variable was the fate of each nest in each interval visit, 0 being a failed interval and 1 a survived interval. One model was performed for each of the four main wader species, of which at least five nests were found in each year. To select the best model, a stepAIC function was applied to select the model with less AIC, and then an ANOVA was performed to compare the selected model (lowest AIC) with the null model, with a Chi-square test (results from the models can be found in ESM 1).
Results
Weather and timing of egg laying
Mean temperature during May was low in 2012 [3.17 °C ± 2.91 (n = 31)], moderate in 2008 and 2010 [4.66 ± 3.32 and 4.05 °C ± 3.49 (n = 31), respectively] and higher in the other years ( > 5 °C). 2010 was a year with much snow in May (Fig. 2), but only in 2012 a large part of the study area was still covered with an extensive amount of snow ( > 60%) upon mean egg laying time as calculated from other years. On 15 June 2012, the area was still covered 63% (Fig. 3).
A positive linear relationship existed between the timing of egg laying and the snow depth in May (ANOVA, F1,610 = 154.27, p < 0.0001). The start of egg laying was delayed by about 2 weeks in 2012, the year with most extensive snow cover far into June (Fig. 3). Timing of egg laying differed also significantly among the species (ANOVA, F3, 610 = 2.78, p = 0.04), with dunlin starting relatively early and red-necked phalarope relatively late (Fig. 3).
Nest fate
From the total number of 567 nests found from the four main species during all the years of study, 29% were depredated, 5% abandoned and 0.9% were lost due to accidentally been trampled by reindeers or flooded. The remaining 64% were successful (Table 1).
The number of nests lost to predators as a proportion of the total number of active nests during the first 2 weeks of incubation was relatively high for 2012, resulting in 32% of nests depredated (Fig. 3). Next came 2008 with 23%, 2013 and 2009 with 16%, 2011 with 13% and 2010 with 9% of nests depredated. The proportion of nests depredated decreased generally per time (week) for all years combined (Paired t test, t42 = − 1.67, p = 0.10) presenting 2008 and 2012 significant negative trends (2008: Paired t test, t6 = 0.01, p = 0.01 and 2012: t5 = − 2.58, p = 0.05).
Annual variation in predators, rodents and survival rate
The numbers of voles and lemmings peaked in 2007 and 2011 (Fig. 4). Lemming numbers in 2011 were the highest since 1980. Lemming and vole numbers were very low the years after the peak years, in 2008–2009 and 2012–2013. In 2008, lemming numbers crashed in early spring before the monitoring of rodents was conducted. Mammalian predator activity was higher after the vole and lemming peak years, in 2008–2009 and 2012–2013. Also in 2011, a relatively large number of predator tracks were found. 2010 was the year with the lowest mammalian predator activity in the study area. However, there was no correlation between the abundance of rodents lagged one year and the predator activity (Pearson correlation, Pearson = 0.718, Paired t test, t4 = 2.065, p = 0.10). The number of breeding pairs of long-tailed skua varied between the years, with 17 breeding pairs in 2007, 30 in 2008, none in 2009, 22 in 2010, 56 in 2011, none in 2012, 2 in 2013, 17 in 2014 and 39 in 2015. There was a high correlation between abundance of long-tailed skua and number of rodents in the area in the same year (Pearson correlation, Pearson = 0.874, Paired t test, t4 = 3.601, p = 0.02).
Abundance of rodents (upper figure, full lines corresponds to field voles and dash line to Norwegian lemmings) and predators (upper figure, dark grey bars represent red foxes and light grey bars stoats). Mean DSR (lower bars) and percentage of successful nests (lower circles) for each year and period and species. Grey colours correspond to different species: black = Eurasian golden plover, dark grey = dunlin, light grey = red-necked phalarope, grey = common redshank
Daily survival rates of wader nests varied markedly between years and species (Fig. 4). Survival rates were lowest in 2012 and highest in 2011. Eurasian golden plovers had relatively low survival rates in all years and common redshanks relatively low survival rate in 2012–2013.
2012 was the only year in which the waders started to breed when a large part of the study area was still covered by snow (Fig. 3). Survival rates were especially low during the first weeks of incubation: 68% of nests survived in 2012, compared to 84.6% in the other years, although the difference was not significant (Paired t test, t42 = − 1.67, p = 0.10).
When analysing the relation of biotic and abiotic factors with the survival rate, we find many differences among each species (Online Resource 1), although the rodent index during the year before and snow depth in May were the dominant factors. For Eurasian golden plover, snow cover was the only significant factor affecting survival. For dunlin, the 1-year-lagged rodent and abundance of long-tailed skuas were the significant factors. Redshank survival seemed to be influenced only by the lagged rodent index, and red-necked phalarope by the snow depth in May.
The number of hatchlings differed significantly between years and species. For every species, 2012 was the year with the fewest hatchlings per nest (Table 2).
Discussion
Nesting success of waders breeding in sub-Arctic southern Lapland was monitored during six years (2008–2013). This included a full cycle of rodent numbers and one season with an extensive snow cover during early spring, which allowed us to infer the effect of ecological and environmental conditions on breeding ecology. Understanding these effects is pivotal as wader breeding ecology is relatively understudied for the sub-Arctic, especially Fennoscandia, despite the fact that these alpine tundra habitats support large numbers of breeding waders (Lindström et al. 2015). Climate change scenarios predict warmer summers but also more precipitation (Radionov et al. 2004). The latter would mean in sub-Arctic areas an increase in snow depth and snow cover, and consequently a shorter breeding season if temperatures during spring remain stable or low (Callaghan et al. 2011).
2012 may be an illustration of the larger variability that Arctic ecosystems may be exposed to in the future, i.e. more frequent extreme events. This was a year with exceptionally large amount of snow during winter, which resulted in the study area being still snow covered at the beginning of the breeding season. Late snow melt had a clear effect on the timing of breeding of the waders. In 2012, the start of incubation was delayed by about 2 weeks compared to the other seasons. Moreover, for waders breeding in the Arctic, a clear relationship between snow cover and timing of breeding was found (Meltofte et al. 2007).
In the Arctic, nesting success of waders is frequently shaped by the regular fluctuations in the abundance of predators and rodents (voles and lemmings) (Rybkin 1998; Ims et al. 2013). Predators, except when rodents are abundant, prey heavily on wader nests. This prey-switching behaviour is known as the alternative prey hypothesis (Roselaar 1979; Summers 1986; Underhill et al. 1993; Ebbinge and Spaans 2002; Quakenbush et al. 2004; Perkins et al. 2007). However, the results of our study suggest that wader breeding success is not a function of lemming or predator abundance in the sub-Arctic, at least when excluding the exceptional breeding season of 2012 (see above). Instrumental in this respect is 2011; despite high lemming numbers (highest numbers since 1980), predation pressure was still relatively high for golden plover and very comparable to the other years. One possible explanation for the difference between Arctic and sub-Arctic ecosystems could be that the red fox, one of the main predators in the study area, is now present in the sub-Arctic (Angerbjörn et al. 2013; Elmhagen et al. 2015). Here, red fox is a more generalist predator typically including more birds in their diet during all phases of the rodent cycle (Elmhagen et al. 2000). They could also move downwards into the valleys when prey is scarce at the tundra, which might completely change the predator–prey interactions. This contrasts to the Arctic where the arctic fox is the main predator, and they cannot so easily switch to a different habitat when rodents are scarce at the tundra, forcing them to focus on wader nests. 2012 was an exceptional year when the negative effects of late snow melt on nesting success was clearly evident. At the beginning of the season, when few snow-free patches were available for nesting, wader nests were easily found by the relatively high number of predators present in the area. Consequently, in 2012 nest predation rates were relatively high and considerable fewer chicks managed to hatch compared to other years. Predation pressure was especially high during the first 2 weeks of the breeding season and much higher than in any other season.
Other studies performed in the Arctic have shown a positive or neutral effect of climate change in the form of higher temperatures and earlier snow melt (Weiser et al. 2018). However, the pattern that we have described in this sub-Arctic seems to be a different scenario, where the effects of climate change could be more precipitation as snow and therefore later snowmelt (Radionov et al. 2004) (Fig. 5).
If indeed the amount of precipitation increases in the sub-Arctic due to global climate change, it is likely that a late snow melt will be much more common in the future (Radionov et al. 2004) if temperatures keep being stable or low during spring. As a consequence, nesting success may become more strongly dependent on the lemming–predator cycle, i.e. a situation more similar to Arctic areas. To test this hypothesis, data on nesting success would also be required for years with a late snow melt and a high abundance of predators and rodents, in order to see whether the alternative prey hypothesis applies in such a situation. In this time series studied, an important event is lacking, a late snow melt season with increasing or intermediate lemming abundance. We presume this event will have obvious consequences in the start of wader breeding and will probably produce higher predation rates; as shown in Meltofte et al. (1981), high snow cover in the beginning of the season despite a high lemming density resulted in high predation. However, if the snow event happens after a lemming crash year with also lower numbers of predators, the effects of predators is unlikely to be high.
The main point here is whether all species could deal with the scenario of a shorter snow-free period during a warmer summer (Radionov et al. 2004). For example, Eurasian golden plovers have a relatively long breeding period, partly because of a long incubation period (Byrkjedal and Thompson 1998), and they might no longer be able to fit their breeding into a shorter season. In addition, higher temperature might change insect phenologies (Tulp and Schekkerman 2008; Koltz et al. 2018), and it is unclear how this would affect chick condition and survival (Machin et al. 2018).
Differences in predation rate between species are observed in this study. Eurasian golden plover is the most preyed species every year except for 2012. It is also the only species that breeds in heathland. This type of habitat might be the easiest to search by foxes and other predators, due to an open vegetation structure.
We have shown that the nesting success of waders breeding in the sub-Arctic is strongly influenced by the timing of snowmelt. An increase in precipitation due to global climate change resulting in a later snowmelt when temperatures in spring are normal or low might thus be negative for these wader populations if these episodes become frequent. However, we also suggest that a late snowmelt might change the interactions between waders, predators and their alternative prey (rodents) in a direction hardly to predict; this makes it very difficult to foresee the exact outcome of global climate change. We recommend running a long-term monitoring scheme of wader breeding success in order to better understand how the ecological and environmental interactions will change over time.
References
Angelstam P, Lindstrom E, Widen P (1984) Role of predation in short-term population fluctuations of some birds and mammals in Fennoscandia. Oecologia 62:199–208
Angerbjörn A, Lundberg H, Tannerfeldt M (2001) Geographical and temporal patterns of lemming population dynamics in Fennoscandia. Ecography 24:298–308
Angerbjörn A, Eide N, Dalén L, Elmhagen B, Hellström P, Ims R, Killengreen S, Landa A, Meijer T, Mela M, Niemimaa J, Norén K, Tannerfeldt M, Yoccoz N, Henttonen H (2013) Conservation in practice: replicated management actions on a large spatial scale. J Appl Ecol 50:59–67
Byrkjedal I, Thompson DBA (1998) Tundra plovers: the Eurasian Pacific and American Golden Plovers and Grey Plover. T & A. D Poyser, London
Callaghan TV, Bergholm F, Christensen TR, Jonasson C, Kokfelt U, Johansson M (2010) A new climate era in the sub-Arctic: accelerating climate changes and multiple impacts. Geophys Res Lett 37:6
Callaghan TV, Johansson M et al (2011) The changing face of Arctic snow cover: A synthesis of observed and projected changes. Ambio 40:17–31
Ebbinge BS, Spaans B (2002) How do Brent Geese (Branta b. bernicla) cope with evil? Complex relationships between predators and prey. J für Ornithol 143:33–42
Elmhagen B, Tannerfeldt M, Verucci P, Angerbjörn A (2000) The arctic fox (Alopex lagopus)—an opportunistic specialist. J Zool 251:139–149
Elmhagen B, Kindberg J, Hellström P, Angerbjörn A (2015) A boreal invasion in response to climate change? Range shifts and community effects in the borderland between forest and tundra. Ambio 44:39–50
European Commission (2013) Natura 2000 barometer. Natura 2000 nature and biodiversity newsletter. European Commission, Brussels, pp 8–9
Gilg O, Hanski I, Sittler B (2003) Cyclic dynamics in a simple vertebrate predator-prey community. Science 302:866–868
Gilg O, Sittler B, Hanski I (2009) Climate change and cyclic predator–prey population dynamics in the high Arctic. Glob Change Biol 15:2634–2652
Hansen J, Schmidt NM, Reneerkens J (2011) Egg hatchability in high Arcticbreeding wader species Charadriiformes is not affected by determining incubation stage using the egg flotation technique. Bird Study 58:522–525
Harrison C, Castell P (2004) Jungvögel, Eier und Nester der Vögel Europas, Nordafrikas und des mittleren Ostens, Aula-Verlag, Wiebelsheim, p 473
Ims RA, Henden J-A, Thingnes AV, Killengreen ST (2013) Indirect food web interactions mediated by predator–rodent dynamics: relative roles of lemmings and voles. Biol Lett 9:20130802
IPCC (2014) Synthesis report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In: Core Writing Team, Pachauri RK, Meyer LA (eds) Climate Change 2014. IPCC, Geneva, p 151
Koltz AM, Schmidt NM, Høye TT (2018) Differential arthropod responses to warming are altering the structure of Arctic communities. R Soc open Sci 5:171503
Labisky RF (1957) Relation of hay harvesting to duck nesting under refuge-pennitee system. J Wildl Manage 21:194–200
Liebezeit JR, Smith PA, Lanctot RB, Schekkerman H, Tulp I, Kendall SJ, Tracy DM, Rodrigues RJ, Meltofte H, Robinson JA, Gratto-Trevor C, McCaffery BJ, Morse J, Zack SW (2007) Assessing the development of shorebird eggs using the flotation method: species-specific and generalized regression models. Condor 109:32–47
Lindén H, Helle E, Helle P, Wikman M (1996) Wildlife triangle scheme in Finland: methods and aims for monitoring wildlife populations. Finn Game Res 49:4–11
Lindström A, Green M, Husby M, Kalas JA, Lehikoinen A (2015) Large scale monitoring of waders on their boreal and arctic breeding grounds in northern Europe. Ardea 103:3–15
MacDonal A, Bolton M (2008) Predation on wader nests in Europe. Ibis 150:54–73
Machín P, Fernández-Elipe J, Klaassen RHG (2018) The relative importance of food abundance and weather on the growth of a subarctic shorebird chick. Behav Ecol Sociobiol 72:42
Mayfield HF (1961) Nesting success calculated from exposure. Wilson Bull 73:255–261
Mayfield HF (1975) Suggestions for calculating nest success. Wilson Bull 87:456–466
McKinnon L, Berteaux D, Bêty J (2014) Predator-mediated interactions between lemmings and shorebirds: a test of the alternative prey hypothesis. Auk 131:619–628
Meltofte H, Elander M, Hjort C (1981) Ornithological observations in Northeast Greenland between 74°30’ and 76°00’N. lat., 1976. Meddelelser om Grønland Biosci 3:52
Meltofte H, Piersma T, Boyd H, McCaffery B, Ganter B, Golovnyuk VV, Graham K, GrattoTrevor CL, Morrison RIG, Nol E, Rösner HU, Schamel D, Schekkerman H, Soloviev MY, Tomkovich PS, Tracy DM, Tulp I, Wennerberg L (2007) Effects of climate variation on the breeding ecology of Arctic shorebirds. Meddelelser om Grønland Biosci 59:1–48
Perkins DE, Smith PA, Gilchrist HG (2007) The breeding ecology of ruddy turnstones (Arenaria interpres) in the eastern Canadian. Arctic Polar Rec 43:135–142
Popova VV (2004) Structure of multi-year variations of the snow cover depth in North Eurasia. Russ Meteorol Hydrol 8:78–88
Quakenbush L, Suydam R, Obritschkewitsch T, Deering M (2004) Breeding biology of Steller’s Eiders (Polysticta stelleri) near Barrow, Alaska, 1991–1999. Arctic 57:166–182
R Development Core Team (2008) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Radionov VF, Aleksandrov EI, Bayborodova VR, Bryazgin NN, Dement’ev AA (2004) Long-term changes of snow cover period in the Arctic. Data Glaciol Stud 97:136–142 (in Russian with English summary)
Riseth JA, Tømmervik H, Helander-Renvall E, Labba N, Johansson C, Malnes E, Bjerke JW, Jonsson C et al (2010) Sámi traditional ecological knowledge as a guide to science: snow, ice and reindeer pasture facing climate change. Polar Rec 47:202–217
Roselaar CS (1979) Fluctuaties in aantallen krombekstrandlopers Calidris ferruginea. Watervogels 4:202–210
Rosenzweig C et al (2007) Assessment of observed changes and responses in natural and managed systems. In: Parry ML, Canziani OF, Palutikof JP, Linden PJ, Hanson CE (eds) Climate change 2007: impacts, adaptation and vulnerability Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, pp 79–131
Rotella JJ, Dinsmore SJ, Shaffer TL (2004) Modeling nest-survival data: a comparison of recently developed methods that can be implemented in MARK and SAS. Anim Biodivers Conserv 27:187–204
Rybkin AV (1998) Lemming density in Taimyr tundra and its influence on reproduction of birds. Int Wader Stud 10:207–213
Serreze MC, Barrett AP, Slater AG, Steele M, Zhang J, Trenberth KE (2007) The large-scale energy budget of the Arctic. J Geophys Res 112:D11122
Shaffer TL (2004) A unified approach to analysing nest success. Auk 121:526–540
Staafjord T (2012) Vindelfjällens naturreservat, Grundutredning om natur, kultur, nyttjande och förvaltning. Nature reserve report Länsstyrelsen Västerbotten. p 171
Stoessel M, Elmhagen B, Vinka M, Hellström P, Angerbjörn A (2017) The fluctuating world of an Arctic predator guild—can winter constraints overrule top-down effects in an alpine tundra ecosystem? Ecography 42:488–499
Summers RW (1986) Breeding production of Darkbellied Brent Geese Branta bernicla bernicla in relation to lemming cycles. Bird Study 33:105–108
Tulp I, Schekkerman H (2008) Has prey availability for arctic birds advanced with climate change? Hindcasting the abundance of tundra arthropods using weather and seasonal variation. Arctic 61:48–60
Underhill LG, Prysjones RP, Syroechkovski EE, Groen NM, Karpov V, Lappo HG, Vanroomen MWJ, Rybkin A, Schekkerman H, Spiekman H, Summers RW (1993) Breeding of waders (Charadrii) and Brent Geese Branta bernicla bernicla at Pronchishcheva Lake, Northeastern Taimyr, Russia, in a peak and a decreasing lemming year. Ibis 135:277–292
van Gils JA, Lisovski S, Lok T, Meissner W, Ożarowska A, de Fouw J, Rakhimberdiev E, Soloviev MY, Piersma T, Klaassen M (2016) Body shrinkage due to Arctic warming reduces red knot fitness in tropical wintering range. Science 352:819–821
Walther G-R (2010) Community and ecosystem responses to recent climate change. Philos Trans R Soc Lond B 365:2019–2024
Walther G-R, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin J-M, Hoegh-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416:389–395
Weiser EM, Lanctot RB, Brown SC, Gates HR et al (2018) Environmental and ecological conditions at Arctic breeding sites have limited effects ontrue survival rates of adult shorebirds. The Auk 135:29–43
Acknowledgements
This research would have been impossible without the continuous encouragement of Martin Green and Åke Lindström. LUVRE project supported us economically during the six years. We thank especially Rob van Bemmelen for all the help and support during the fieldwork campaigns. During the six years of work, many people have been involved with this project. Thanks to all for many shared moments in the cold and sometimes too hot tundra.
Funding
Accommodation at Vindelfjällen Research Station and travel expenses were covered by the LUVRE project (Lund University).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. The fieldwork was carried out under permits from the Lund/Malmö Ethical Committee for Animal Experiments (M160-11, M27-10, M33-13).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Machín, P., Fernández-Elipe, J., Hungar, J. et al. The role of ecological and environmental conditions on the nesting success of waders in sub-Arctic Sweden. Polar Biol 42, 1571–1579 (2019). https://doi.org/10.1007/s00300-019-02544-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-019-02544-x