Abstract
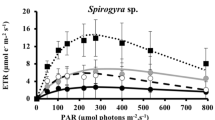
The photosynthetic characteristics through P-E curves and the effect of UV radiation on photosynthesis (measured as rapid adjustment of photochemistry, F v/F m) and DNA damage (as formation of CPDs) were studied in field specimens of green, red and brown algae collected from the eulittoral and sublittoral zone of Fildes Peninsula (King George Island, Antarctic). The content of phenolic compounds (phlorotannins) and the antioxidant activity were also studied in seven brown algae from 0 to 40 m depth. The results indicated that photosynthetic efficiency (α) was high and did not vary between different species and depths, while irradiances for saturation (E k) averaged 55 μmol m−2 s−1 in subtidal and 120 μmol m−2 s−1 in eulittoral species. The studied species exhibited notable short-term UV tolerance along the vertical zonation. In intertidal and shallow water species, decreases in F v/F m by UV radiation were between 0 and 18 %, while in sublittoral algae, decreases in F v/F m varied between 3 and 35 % relative to PAR treatment. In all species, recovery was high averaging 84–100 %. The formation of CPDs increased (15–150 %) under UV exposure, with the highest DNA damage found in some subtidal species. Phlorotannin content varied between 29 mg g−1 DW in Ascoseira mirabilis from 8 m depth and 156 mg g−1 DW in Desmarestia menziesii from 17 m depth. In general, phlorotannin concentrations were constitutively high in deeper sublittoral brown algae, which were correlated with higher antioxidant activities of algal extracts and low decreases in photosynthesis. UV radiation caused a strong decrease in phlorotannin content in the deep-water Himantothallus grandifolius, whereas in D. menziesii and Desmarestia anceps, induction of the synthesis of phlorotannins by UV radiation was observed. The antioxidant activity was in general less affected by UV radiation.






Similar content being viewed by others
References
Aguilera J, Bischof K, Karsten U, Hanelt D, Wiencke C (2002) Seasonal variation in ecological patterns in macroalgae from an Arctic fjord. II. Pigment accumulation and biochemical defence systems against high light stress. Mar Biol 140:1087–1095
Amsler CD, Iken K, McClintock JB, Amsler MO, Peters KJ, Hubbard JM, Furrow FB, Baker BJ (2005) Comprehensive evaluation of the palatability and chemical defenses of subtidal macroalgae from the Antarctic Peninsula. Mar Ecol Prog Ser 294:141–159
Arnold TM (2003) To grow and defend: lack of tradeoffs for brown algal phlorotannins. Oikos 100:406–408
Becker S, Graeve M, Bischof K (2010) Photosynthesis and lipid composition of the Antarctic endemic rhodophyte Palmaria decipiens: effects of changing light and temperature levels. Polar Biol 33:945–955
Bertness MD, Crain CM, Silliman BR, Bazterrica MC, Reyna MV, Hildago F, Fariña JK (2006) The community structure of western Atlantic Patagonian rocky shores. Ecol Monogr 76:439–460
Bischof K, Hanelt D, Wiencke C (1998a) UV-radiation can affect depth-zonation of Antarctic macroalgae. Mar Biol 131:597–605
Bischof K, Hanelt D, Tüg H, Karsten U, Brouwer PEM, Wiencke C (1998b) Acclimation of brown algal photosynthesis to ultraviolet radiation in Arctic coastal waters (Spitsbergen, Norway). Polar Biol 20:388–395
Bischof K, Rautenberger R, Brey L, Pérez-Lloréns JL (2006) Physiological acclimation to gradients of solar irradiance within mats of the filamentous green macroalga Chaetomorpha linum from southern Spain. Mar Ecol Prog Ser 306:165–175
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol 28:25–30
Caldwell MM, Robberecht R, Flint SD (1983) Internal filters: prospects for UV-acclimation in higher plants. Physiol Plant 58:445–450
Connan S, Delisle F, Deslandes E, Ar Gall E (2006) Intra-thallus phlorotannin content and antioxidant activity in Phaeophyceae of temperate waters. Bot Mar 49:39–46
Cruces E, Huovinen P, Gómez I (2012) Phlorotannin and antioxidant responses upon short-term exposure to UV radiation and elevated temperature in three South Pacific kelps. Photochem Photobiol 88:58–66
Cruces E, Huovinen P, Gómez I (2013) Interactive effects of UV radiation and enhanced temperature on photosynthesis, phlorotannin induction and antioxidant activities of two sub-Antarctic brown algae. Mar Biol 160:1–13
de la Coba F, Aguilera J, Figueroa FL, de Gálvez MV, Herrera E (2009) Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. J Appl Phycol 21:161–169
Drew EA, Hastings RM (1992) A year-round ecophysiological study of Himantothallus grandifolius (Desmarestiales, Phaeophyta) at Signy Island, Antarctica. Phycologia 31:262–277
Dubois A, Iken K (2012) Seasonal variation in kelp phlorotannins in relation to grazer abundance and environmental variables in the Alaskan sublittoral zone. Algae 27:9–19
Fairhead VA, Amsler CD, McClintock JB, Baker BJ (2005) Variation in phlorotannin content within two species of brown macroalgae (Desmarestia anceps and D. menziesii) from the Western Antarctic Peninsula. Polar Biol 28:680–686
Fairhead VA, Amsler DA, McClintock JB (2006) Lack of defence or phlorotannin induction by UV radiation or mesograzers in Desmarestia anceps and D. menziesii (Phaeophyceae). J Phycol 42:1174–1183
Franklin LA, Yakovleva I, Karsten U, Lüning K (1999) Synthesis of mycosporine-like amino acids in Chondrus crispus (Florideophyceae) and the consequences for sensitivity to ultraviolet B radiation. J Phycol 35:682–693
Gambi MC, Lorenti M, Russo GF, Scipione MB (1994) Benthic associations of the shallow hard bottoms off Terra Nova Bay, Ross Sea: zonation, biomass and population structure. Antarct Sci 6:449–462
Gómez I, Huovinen P (2010) Induction of phlorotannins during UV exposure mitigates inhibition of photosynthesis and DNA damage in the kelp Lessonia nigrescens. Photochem Photobiol 86:1056–1063
Gómez I, Huovinen P (2011) Morpho-functional patterns and zonation of South Chilean seaweeds: the importance of photosynthetic and bio-optical traits. Mar Ecol Prog Ser 422:77–91
Gómez I, Weykam G, Klöser H, Wiencke C (1997) Photosynthetic light requirements, daily carbon balance and zonation of sublittoral macroalgae from King George Island (Antarctica). Mar Ecol Prog Ser 148:281–293
Gómez I, Wulff A, Roleda MY, Huovinen P, Karsten U, Quartino L, Dunton K, Wiencke C (2009) Light and temperature demands of marine benthic microalgae and seaweeds in the polar regions. Bot Mar 52:593–608
Gutkowski R, Maleszewski S (1989) Seasonal changes of the photosynthetic capacity of the Antarctic macroalga Adenocystis utricularis (Bory) Skottsberg. Polar Biol 10:145–148
Hanelt D, Jaramillo MJ, Nultsch W, Senger S, Westermeier R (1994) Photoinhibition as a regulative mechanism of photosynthesis in marine algae of Antarctica. Ser Cient Inst Antart Chil 44:67–77
Hanelt D, Wiencke C, Nultsch W (1997a) Influence of UV radiation on photosynthesis of Arctic macroalgae in the field. J Photochem Photobiol B Biol 38:40–47
Hanelt D, Wiencke C, Karsten U, Nultsch W (1997b) Photoinhibition and recovery after high light stress in different developmental and life-history stages of Laminaria saccharina (Phaeophyta). J Phycol 33:387–395
Hanelt D, Melchersmann B, Wiencke C, Nultsch W (1997c) Effects of high light stress on photosynthesis of polar macroalgae in relation to depth distribution. Mar Ecol Prog Ser 149:255–266
Holzinger A, Di Piazza L, Lütz C, Roleda MY (2011) Sporogenic and vegetative tissues of Saccharina latissima (Laminariales, Phaeophyceae) exhibit distinctive sensitivity to experimentally enhanced ultraviolet radiation: photosynthetically active radiation ratio. Phycol Res 59:221–235
Hoyer K, Karsten U, Wiencke C (2002) Induction of sunscreen compounds in Antarctic macroalgae by different radiation conditions. Mar Biol 141:619–627
Huovinen P, Gómez I (2011) Spectral attenuation of solar radiation in Patagonian fjord and coastal waters and implications for algal photobiology. Cont Shelf Res 31:254–259
Huovinen P, Leal P, Gómez I (2010) Interacting effects of copper, nitrogen and ultraviolet radiation on the physiology of three south Pacific kelps. Mar Freshw Res 61:330–341
Jassby AD, Platt T (1976) Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol Oceonogr 21:540–547
Johansson G, Snoeijs P (2002) Macroalgal photosynthetic responses to light in relation to thallus morphology and depth zonation. Mar Ecol Prog Ser 244:63–72
Jones LW, Kok B (1966) Photoinhibition of chloroplast reactions. I. Kinetics and action spectra. Plant Physiol 41:1037–1043
Karentz D, McEuen FS, Land MC, Dunlap WC (1991) Survey of mycosporine-like amino acid compounds in Antarctic marine organisms: potential protection from ultraviolet exposure. Mar Biol 108:157–166
Kjeldstad B, Frette Ø, Erga SR, Browman HI, Kuhn P, Davis R, Miller W, Stamnes JJ (2003) UV (280–400 nm) optical properties in a Norwegian fjord system and an intercomparison of underwater radiometers. Mar Ecol Prog Ser 256:1–11
Klöser H, Ferreyra G, Schloss I, Mercuri G, Laturnus F, Curtosi A (1993) Seasonal variation of algal growth conditions in sheltered Antarctic bays: the example of Potter Cove (King Goerge Island, South Shetlands). J Mar Syst 4:289–301
Klöser H, Mercuri G, Laturnus F, Quartino ML, Wiencke C (1994) On the competitive balance of macroalgae at Potter Cove (King George Island, South Shetlands). Polar Biol 14:11–16
Klöser H, Quartino ML, Wiencke C (1996) Distribution of macroalgae and macroalgal communities in gradients of physical conditions in Potter Cove, King George Island, Antarctica. Hydrobiologia 333:1–17
Lüder UH, Clayton MN (2004) Induction of phlorotannins in the brown macroalga Ecklonia radiata (Laminariales, Phaeophyta) in response to simulated herbivory—the first microscopic study. Planta 218:928–937
Lüder U, Knoetzel J, Wiencke C (2001) Acclimation of photosynthesis and pigments to seasonally changing light conditions in the endemic Antarctic red macroalga Palmaria decipiens. Polar Biol 24:598–603
Lüning K, Dring MJ (1985) Action spectra and spectral quantum yield of photosynthesis in marine macroalgae with thin and thick thalli. Mar Biol 87:119–129
Mori T, Nakane M, Hattori T, Matsunaga T, Ihara M, Nikaido O (1991) Simultaneous establishment of monoclonal antibodies specific for either cyclobutane pyrimidine dimer or (6–4) photoproduct from the same mouse immunized with ultraviolet-irradiated DNA. Photochem Photobiol 54:225–232
Pavia H, Cervin G, Lindgren A, Aberg P (1997) Effects of UV-B radiation and simulated herbivory on phlorotannins in the brown alga Ascophyllum nodosum. Mar Ecol Prog Ser 157:139–146
Pérez-Rodríguez E, Aguilera J, Gómez I, Figueroa FL (2001) Excretion of coumarins by the Mediterranean alga Dasycladus vermicularis in response to environmental stress. Mar Biol 139:633–639
Ragan MA, Glombitza KW (1986) Phlorotannins, brown algal polyphenols. In: Round FE, Chapman DJ (eds) Progress in phycological research. Biopress, Bristol, pp 129–241
Ramírez ME, Villouta ES (1984) Distribución de algas intermareales en Cabo Melville, Isla Rey Jorge, Shetland del Sur, Territorio Antártico Chileno. Ser Cient Inst Antart Chil 31:59–166
Rautenberger R, Bischof K (2006) Impact of temperature on UV-susceptibility of two Ulva (Chlorophyta) species from Antarctic and Subantarctic regions. Polar Biol 29:988–996
Richardson MG (1979) The distribution of Antarctic marine macroalgae related to depth and substrate. Br Antarct Surv Bull 49:1–13
Richter A, Wuttke S, Zacher K (2008) Two years of in situ UV measurements at the Dallmann Laboratory/Jubany Station. Ber Polarforsch Meeresforsch 571:12–19
Roleda MY, Hanelt D, Wiencke C (2005) Growth kinetics related to physiological parameters in young Saccorhiza dermatodea and Alaria esculenta sporophytes exposed to UV radiation. Polar Biol 28:539–549
Roleda MY, Wiencke C, Hanelt D (2006) Thallus morphology and optical characteristics affect growth and DNA damage by UV radiation in juvenile Arctic Laminaria sporophytes. Planta 223:407–417
Roleda MY, Zacher K, Wulff A, Hanelt D, Wiencke C (2007a) Photosynthetic performance, DNA damage and repair in gametes of the endemic Antarctic brown alga Ascoseira mirabilis exposed to ultraviolet radiation. Austral Ecol 32:917–926
Roleda MY, Wiencke C, Hanelt D, Bischof K (2007b) Sensitivity of early life stages of macroalgae from the northern hemisphere to ultraviolet radiation. Photochem Photobiol 83:851–862
Roleda MY, Zacher K, Wulff A, Hanelt D, Wiencke C (2008) Susceptibility of spores of different ploidy levels from antarctic Gigartina skottsbergii (Gigartinales, Rhodophyta) to ultraviolet radiation. Phycologia 47:361–370
Roleda MY, Campana G, Wiencke C, Hanelt D, Quartino ML, Wulff A (2009) Sensitivity of Antarctic Urospora penicilliformis (Ulotrichales, Chlorophyta) to ultraviolet radiation is life stage dependent. J Phycol 45:600–609
Roleda MY, Lütz-Meindl U, Wiencke C, Lütz C (2010) Physiological, biochemical, and ultrastructural responses of the green macroalga Urospora penicilliformis from Arctic Spitsbergen to UV radiation. Protoplasma 243:105–116
Schoenwaelder MEA (2002) The occurrence and cellular significance of physodes in brown algae. J Phycol 41:125–139
Schreiber U, Bilger W, Neubauer C (1994) Chlorophyll fluorescence as a non-intrusive indicator for rapid assessment of in vivo photosynthesis. Ecol Stud 100:49–70
Schwarz A-M, Hawes I, Andrew N, Norkko A, Cummings V, Thrush S (2003) Macroalgal photosynthesis near the southern global limit for growth; Cape Evans, Ross Sea, Antarctica. Polar Biol 26:789–799
Schwarz A-M, Hawes I, Andrew N, Mercer S, Cummings V, Thrush S (2005) Primary production potential of non-geniculate coralline algae at Cape Evans, Ross Sea, Antarctica. Mar Ecol Prog Ser 294:131–140
Setlow RB (1974) The wavelengths in sunlight effective in producing skin cancer: a theoretical analysis. Proc Natl Acad Sci USA 71:3363–3366
Skene KR (2004) Key differences in photosynthetic characteristics of nine species of intertidal macroalgae are related to their position on the shore. Can J Bot 82:177–184
Smale DA (2008) Continuous benthic community change along a depth gradient in Antarctic shallows: evidence of patchiness but not zonation. Polar Biol 31:189–198
Swanson AK, Druehl LD (2002) Induction, exudation and potential UV protective role of kelp phlorotannins within coastal waters. Aquat Bot 1578:1–13
Swanson AK, Fox C (2007) Altered kelp (Laminariales) phlorotannins and growth under elevated carbon dioxide and ultraviolet-B treatments can influence associated intertidal food webs. Glob Change Biol 13:1696–1709
Tao C, Sugawara T, Maeda S, Wang X, Hirata T (2008) Antioxidative activities of a mycosporine-like amino acid, porphyra-334. Fish Sci 74:1166–1172
Weykam G, Gómez I, Wiencke C, Iken K, Klöser H (1996) Photosynthetic characteristics and C:N ratios of macroalgae from King George Island (Antarctica). J Exp Mar Biol Ecol 204:1–22
Wiencke C, Roleda MY, Gruber A, Clayton MN, Bischof K (2006) Susceptibility of zoospores to UV radiation determines upper depth distribution limit of Arctic kelps: evidence through field experiments. J Ecol 94:455–463
Zacher K, Roleda MY, Hanelt D, Wiencke C (2007a) UV effects on photosynthesis and DNA in propagules of three Antarctic seaweeds (Adenocystis utricularis, Monostroma hariotii and Porphyra endiviifolium). Planta 225:1505–1516
Zacher K, Wulff A, Molis M, Hanelt D, Wiencke C (2007b) Ultraviolet radiation and consumer effects on a field-grown intertidal macroalgal assemblage in Antarctica. Glob Change Biol 13:1201–1215
Zacher K, Rautenberger R, Hanelt D, Wulff A, Wiencke C (2009) The abiotic environment of polar marine benthic algae. Bot Mar 52:483–490
Zielinski K (1990) Bottom macroalgae of the Admiralty Bay (King George Island, Antarctica). Pol Polar Res 11:95–131
Acknowledgments
The study was supported by the Grant T-20-09 from Instituto Antártico Chileno (INACH). We thank the technical assistance of M. Orostegui and C. Rosas in the laboratory analyses. We are also grateful to D. Schories, I. Garrido, J. Holtheuer and I. del Moral for field assistance and scuba diving.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huovinen, P., Gómez, I. Photosynthetic characteristics and UV stress tolerance of Antarctic seaweeds along the depth gradient. Polar Biol 36, 1319–1332 (2013). https://doi.org/10.1007/s00300-013-1351-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-013-1351-3