Abstract
The marine ecosystem in Kongsfjorden (79°N), a glacial fjord in Svalbard, is to a large extent well known with regard to hydrography, mesozooplankton and higher trophic levels. Research on primary production and lower trophic levels is still scare and especially investigations from winter and spring periods. The spring bloom dynamics in Kongsfjorden were investigated in 2002. The development in nutrient conditions, phytoplankton, protozoans and primary production were followed from 15 April until 22 May. The winter/spring in 2002 was categorized as a cold year with sea ice cover and water masses dominated by local winter-cooled water. The spring bloom started around 18 April and lasted until the middle of May. The bloom probably peaked in late April, but break-up of sea ice made it impossible to sample frequently in this period. Diatoms dominated the phytoplankton assemblage. We estimated the total primary production during the spring bloom in 2002 to range 27–35 g C m−2. There was a mismatch situation between the mesozooplankton and the phytoplankton spring bloom in 2002.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A large part of the annual primary production in many temporal and Arctic marine ecosystems occurs during spring (Sakshaug 2004) and is important in providing energy to marine food webs. Especially in the Arctic, after the long polar night, the input of energy to the marine ecosystem is important. Light is very often the limiting factor for phytoplankton growth during winter and spring in Arctic areas due to ice cover and snow, and therefore the break-up of sea ice and the depth of vertical mixing are important factors for the onset of the spring bloom (Sakshaug et al. 2009). This period is however rarely studied in the Arctic due to logistical challenges. To learn more about the onset of the productive season, production measurements are essential as biomass build-up seen as high Chl-a can be delayed depending on grazing pressure. Diatoms are one of the most important phytoplankton groups during the spring bloom, although the haptophyte Phaeocystis pouchetii often co-occurs as single cells or colonies (Eilertsen et al. 1981; Wassmann et al. 1999; Degerlund and Eilertsen 2010). How secondary producers utilize the phytoplankton biomass during the spring bloom has a major influence on the fate of the production (Cushing 1992; Reigstad et al. 2000). Either it will be grazed and recycled in the upper water masses or it may sink to deeper waters (Wassmann 1998). Recent research has also emphasized the importance of nanoplankton and picoplankton (Lovejoy et al. 2007; Sakshaug et al. 2009; Rokkan Iversen and Seuthe 2010) and showed that the concept of short, low-diversity polar food chains is overly simplistic (Smetacek and Nicol 2005).
Kongsfjorden is a glacial fjord in the Arctic situated on the west coast of Spitsbergen in the Svalbard archipelago (74–81°N). Relatively warm Atlantic water (AW, >3°C) is carried along the west coast of Spitsbergen by the west Spitsbergen current (WSC) and is advected into Kongsfjorden at irregular intervals where it mixes with the colder local water (LW, −0.5 to 1.0°C) (Svendsen et al. 2002; Cottier et al. 2005). The advection of Atlantic water across the shelf during summer, changes the water mass inside the fjord from Arctic dominance in winter to Atlantic dominance in summer (Svendsen et al. 2002). These advective processes affect all parts of the marine ecosystem inside the fjord (Hop et al. 2002). Major inter-annual variations in ocean temperature have been registered by Cottier et al. (2005). They demonstrated that the fjord water showed distinct inter-annual variability in the heat content and in the timing and duration of the advective period, giving rise to the concept of warm and cold years. This is likely related to the temperature conditions in the WSC (Walczowski and Piechura 2006, 2007), which extent reflects the heat content transported to the Arctic in a branch of the North Atlantic Current. Atlantic and Arctic water masses are then mixed on the shelf of West Spitsbergen and advected into Kongsfjorden as transformed Atlantic water (Svendsen et al. 2002).
Hop et al. (2002) reviewed the knowledge about the marine ecosystem of Kongsfjorden and pointed out the main gaps. In general, quantitative data on production, biomass and consumption of phytoplankton were lacking from the pelagic ecosystem in Kongsfjorden. Several later studies have focused on some of these gaps and provided information about phytoplankton species (Okolodkov et al. 2000; Wiktor and Wojciechowska 2005; Leu et al. 2006; Hegseth and Tverberg 2008; Piwosz et al. 2009; Wang et al. 2009; Rokkan Iversen and Seuthe 2010; Seuthe et al. 2010), on bacterial abundances and communities (Janowska et al. 2005; Piquet et al. 2010; Rokkan Iversen and Seuthe 2010) and on zooplankton (Basedow et al. 2004; Lischka and Hagen 2005, 2007; Willis et al. 2006; Lischka et al. 2007; Narcy et al. 2009; Walkusz et al. 2009). Basic information about primary production in Kongsfjorden is limited and only few measurements have been published (Eilertsen et al. 1989; Hop et al. 2002; Piwosz et al. 2009; Rokkan Iversen and Seuthe 2010). Of those, only one includes the spring period (Rokkan Iversen and Seuthe 2010), with only one measurement per month.
The aim of this work was to investigate the timing and development of the spring bloom at higher time resolution, with focus on nutrients, primary production, phytoplankton and protozoans (ciliates and dinoflagellates), related to environmental conditions in Kongsfjorden during spring 2002.
Materials and methods
Locality and sampling
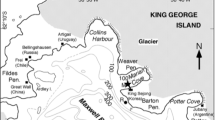
Sampling was done at Station 26/Kb3 (78°54′N 12°00′E) in Kongsfjorden, west Spitsbergen in 2002 (Fig. 1). Nitrate, silicate, phosphate, chlorophyll-a (Chl-a), biogenic silica, particulate organic carbon and samples of phytoplankton and protozoans were collected in the upper water layer (Table 1). Samples from 15 April and 18 April were collected from RV Lance using Niskin bottles mounted on a CTD (Seabird Electronics SBE9plus) at 2, 5, 10, 25, 50, 75, 100, 150 and 200 m depth. Samples from the remaining period until 22 May were collected from a small boat using a 1.5-L plastic non-toxic water collector (VWR International) at 0, 5, 10 and 20 m depth. On 16 and 22 May, additional samples were taken at 30, 40 and 60 m depth. No samples were collected between 18 April and 1 May because of difficult ice conditions. Physical data (salinity, temperature and sigma-t) were recorded with a small transportable CTD (SD204 SAIV A/S).
Schematic overview of the main current system around Spitsbergen, with the West Spitsbergen Current (black arrow) transporting warm Atlantic water along the west coast of Spitsbergen and the East Spitsbergen Current (dotted arrow) transporting cold Arctic water along the east coast of Spitsbergen. Samples were taken at Station Kb3 in Kongsfjorden. Figure was adopted from Seuthe et al. (2010)
Nutrients
Dissolved silicate and phosphate were analyzed in triplicates less than 6 h after collection according to Strickland and Parsons (1972). Subsamples for nitrate analysis were frozen at −18°C in 200-ml polyethylene bottles until analysis 5 month later according to Strickland and Parsons (1972). As long as the difference in absorbance was <0.01 (±0.25 mmol m−3), only two parallels for nitrate were analyzed.
Biomass
For Chl-a analysis, 100–270 ml sea water was filtered on 0.7-μm glass fiber filters (Advantec MFS Inc.) in triplicates, less than 6 h after collection. Filters were immediately extracted in pure methanol for 4–8 h in the dark at room temperature and measured using a Turner fluorometer calibrated with a Chl-a standard (Sigma c 6144) according to (Parsons et al. 1984). For biogenic silica, 250–2,000 ml sea water were filtered on 0.6 μm Poretrics polycarbonate filter (Osmonics Inc.) and frozen at −18°C less than 6 h after sampling. Further analyses were done after 3 months using Na2CO3 hydrolyses (Paasche 1980). Water samples for particulate organic carbon and nitrogen (POC, PON) were filtered on precombusted Whatmann GF/F glass fiber filters and frozen at −18°C. The filters were unfrozen, dried at 60°C, and fumed with HCl for 24 h before analysis 7 month later, using a 440-elemental analyzer (Leeman lab, CEC). Carbon biomass for phytoplankton was calculated from Chl-a concentrations using a range of POC/Chl-a ratios (20–100). The regression between Chl-a and POC in our investigation (data not shown) gave a very high ratio (POC/Chl-a = 290) most likely because no POC samples were taken before and during the peak of the bloom, and the POC was probably dominated by detritus and/or heterotrophic organisms. We have chosen to calculate the phytoplankton biomass using a range of this ratio (20–100), to recognize the highly variable content of Chl-a in phytoplankton cells. The integrated values of Chl-a and POC were calculated using trapezoidal integration of values from 0, 5, 10 and 20 m depth.
Phytoplankton and protozoans (ciliates and dinoflagellates)
Semi-quantitative analysis of phytoplankton was done from water samples preserved with Lugol’s iodine to a final concentration 0.2–0.3%. After 9 months in darkness at 4°C, subsamples were sedimented for 24 h in 50 ml sedimentation chambers before phytoplankton cells >20 μm and Phaeocystis colonies were counted using an inverted microscope. Ciliates and dinoflagellates (protozoans) were counted at DMU (Danmarks miljøundersøgelser) Roskilde, Denmark, from samples fixed with Lugol’s iodine. After 24 months in darkness, 50 ml were settled for at least 24 h before counting using an inverted microscope. Samples for analysis of phytoplankton and protozoa were stored for longer time than what is optimal. It is therefore likely that some of the material could have degraded before counting 9 and 24 months after sampling. Protozoans were categorized and divided into size-classes with 10-μm intervals. The cells were size-measured by length and width to determine the biovolume, and the carbon content was estimated as pg C cell−1 = 0.76 pg C * cell volume0.819 for both dinoflagellates and ciliates according to (Menden–Deuer and Lessard 2000). The integrated values of protozoan biomass on 1 and 4 May were calculated using the biomass at 5 m depth as an average for the upper 30 m (only one sample was taken at those dates). Evaluating the uncertainty using this approach, data from 16 and 22 May shows that the integrated biomass would vary with 12% if only the value from 5 m depth were used instead of all the measured depths. The integrated value of protozoan biomass on 16 May was calculated from the biomass at 5 and 20 m, and the integrated value on 22 May was calculated from the biomass at 0, 5, 10 and 20 m.
Primary production
Particulate primary production (hereafter named primary production) was measured in situ using the 14C method (Parsons et al. 1984). Water samples from 0, 5 and 10 m were incubated in situ in three light and one dark polycarbonate bottles at the respective depths for 24 h with a concentration of 462.5 Bq ml−1 added as NaH14CO3. Samples were filtered using gentle vacuum and filters were frozen immediately thereafter. For analyses, the filters were thawed, acid fumed and 18 ml of Ultima Gold™ XP (Packard) was added before counting on a liquid scintillation counter (MINAX β 4000 series, Packard). Disintegrations per minute (DPM) were calculated from counts per minute (CPM) and a Quench curve. The dark bottle values were subtracted from the light bottle values and the production rates were calculated assuming that the total CO2 concentration was 2.05 mM (Gargas 1975). Integrated primary production was calculated for the depth interval 0–30 m and 0–40 m assuming no production at 30 and 40 m, respectively, and a linear decrease from 10 to 30 and 40 m, respectively. The primary production rate from 20 and 25 m were used as an average for the depth interval 10–30 m and 10–40 m, respectively, while trapezoid integration was used on the values from 0–10 m with 2.5 m intervals. The estimate of total primary production during the spring 2002 was based on some additional assumptions and calculations: The spring bloom was assumed to last from 18 April until 13 May. Production was integrated to both 30 and 40 m. Nitrate consumption was used to calculate the production from 18 April to 30 April (when no primary production measurements were done) and measured primary production rates were used to calculate the production from 1 May to 13 May. The start concentration of nitrate was assumed to be 7.5 μM based on the concentration at 50 m on 18 April. The Redfield ratio C:N = 6.63 is used to convert from μM nitrate to μM carbon (Redfield 1934). An average of the integrated primary production rates from the first 2 weeks of May (0.685–0.85 g C m−2 day−1, n = 4) was used to calculate the primary production from 1 May to 13 May.
Results
Ice conditions
Between 15 and 18 April, warmer weather and strong wind broke up the sea ice at the sampling station and moved it out of the fjord (Fig. 2). During this period, samples were collected from R/V Lance. The ice was advected back into the fjord when the wind direction changed around 20 April. The sea ice stayed inside the fjord until 29 April, when the ice broke up again and was transported out of the fjord. There was still ice in the inner part of the fjord by the end of May. Only a thin layer of snow covered the sea ice (<2 cm, personal observations). The major wind directions in the sampling period were from east and southeast. From April 15 2002, Kongsfjorden experienced midnight sun and the day length had increased 24 h within 2 months (http://www.esrl.noaa.gov/gmd/grad/solcalc/).
Wind speed, air temperature and photosynthetically active radiation (PAR) in the air from 10 April to 30 May in Ny-Ålesund, Svalbard. Negative wind speed values indicate wind directions from 270° to 360° (in fjord) and positive wind speed values indicate wind directions from 90° to 180° (out fjord). Gray boxes indicate periods with ice at Station Kb3. PAR data is plotted with 4 h intervals starting at midnight. Wind speed data, wind direction data and temperature data were obtained from the Norwegian Metrological Institute (DNMI) in Tromsø. Data on PAR in the air was measured on the roof of the Sverdrup research Station in Ny-Ålesund and was provided by the Sverdrup Research Station (Norwegian Polar Institute), Ny-Ålesund. The ice condition data is from own observations
Hydrographic conditions
On 15 April, the hydrographical conditions were characterized by homogeneous water masses (−1.8°C and 34.6 salinity) without any stratification (Fig. 3). From 18 April and onward, a weak thermal stratification developed in the top 35 m and the water column was characterized by a weakly increasing density with depth. Throughout the period, the thermal stratification was disrupted several times. The water temperature increased during the period but never exceed 0°C. Salinity was around 34.2 at the sampled depths on 10 May, but from 10 to 13 May it dropped to 33.6, which indicated a shift to different water masses at the station. It stayed in this range until last sampling on 22 May (Fig. 3). Air temperatures were still well below 0°C during most of May (Fig. 1). Thus, local melting or run-off did probably not contribute significantly to this shift in salinity.
Nutrients and chlorophyll-a
Nitrate and silicate concentrations were high on 15 and 18 April, 6.3 μM and 4.6–4.7 μM, respectively (Table 2; Fig. 4). The silicate concentrations on 15 April were homogenous down to at least 200 m, with values between 4.5 and 4.8 μM indicating winter values (Table 2). The depth profile of nitrate on 18 April showed a slight decrease in nitrate concentrations in the top 25 m compared to the concentration at 50 m, with 5.8–6.2 μM and 7.5 μM, respectively. Silicate did not show the same patterns of surface decrease (Table 2). Between 18 April and 1 May, the Chl-a concentration increased and the nutrient concentrations decreased considerably (Fig. 4a, b). The highest concentration of Chl-a was measured at 20 m on 1 May (2 mg m−3). While the inorganic silicate decreased, the biogenic silica increased showing the incorporation of silicate into diatom frustules. The total silicate (silicate + biogenic silica) decreased in the top 20 m throughout the period (data not shown) indicating that diatom frustules were removed from the top 20 m. There was a significant (P < 0.05) positive correlation between the concentration of Chl-a and biogenic silica (data not shown). Except for 15 and 18 April and 4 May, the Chl-a concentrations increased slightly with depth (Table 2). Between 1 and 4 May, there was an episodic increase in nutrients in the upper layer, most pronounced at 20 m depth, where the nitrate concentration increased from 0.1 to 5.7 μM (Table 2). This was probably caused by the erosion of the weak thermal stratification and convection of nitrate from deeper waters. Silicate did not increase during the same period, which can be caused by the fact that silicate, in the form of diatom frustules (as mention above), was sinking out of the surface water while nitrogen to a larger extent are being recycled in the surface layers. On 8 May, the nitrate concentration at 20 m depth had decreased to 0.6 μM. Phosphate was measured tree times during the first 2 weeks of May ranging from 0.04 (±0.02) to 0.55 (±0.29) μM, and was highest at 0 m on 1 May (data not shown).
Species and biomass
Diatoms dominated the larger phytoplankton during the spring bloom. However, the dominant species changed during the 5-week period (Fig. 5). The succession changed from a Fragilariopsis spp. dominated community in April to a Chaetoceros spp. dominated community in early May. From 4 to 13 May, the larger Thalassiosira spp. dominated. Phaeocystis pouchetii colonies were mainly present after 13 May. Athecate dinoflagellates dominated the protozoans both in abundance and in biomass, and the protozoan biomass was generally dominated by cells >20 μm (Fig. 6). Aloricate forms dominated the ciliate assemblage. Most species were considered to be heterotrophic or mixotrophic. Only Mesodinium sp. was considered autotrophic, but constituted <6% of the total protozoan biomass at all times. In the beginning of May, the integrated biomass of protozoans corresponded to 22–38% of the integrated phytoplankton biomass (Table 3). By the end of May, the situation changed and the integrated protozoan biomass was higher than that of phytoplankton.
Primary production
Primary production rates followed the development in phytoplankton biomass from 1 to 22 May (Fig. 4b). The highest primary production (93 ± 2 mg C m−3 day−1) was measured at the surface on 1 May and remained high at the surface through 8 May. Later in May, primary production was higher at 5 or 10 m than at the surface. Integrated primary production was highest on 1 May ranging 1.52–1.85 g C m−2 day−1 (30–40 m), and decreased during May (Table 3). Average for the investigated period was 0.47–0.58 g C m−2 day−1 (n = 6). Total primary production during the periods 18–30 April and 1–13 May were estimated to be 18–24 g C m−2 and 9–11 g C m−2, respectively. In total, that gives an estimate of primary production during spring in 2002 of 27–35 g C m−2. The assimilation index (calculated as mg Chl-a m−3/mg C m−3 day−1), is a measure of the physiological state of the phytoplankton community and decreased with depth (data not shown). Based on integrated values of Chl-a and primary production (Table 3), the assimilation index ranged from 14 to 36 (mg C (mg Chl-a)−1 day−1) during May.
Discussion
Water masses and ice conditions
When sampling started on 15 April, the sampling station was ice covered and the water column was well mixed with homogeneous winter cooled water (WCW). This is also supported by the investigation by Walkusz et al. (2009), who found homogeneous water masses throughout the fjord during spring 2002. Since melting of ice and run-off from land usually do not start before June/July (Svendsen et al. 2002) no strong density stratification developed during April and May. In many north Norwegian fjords, spring blooms have developed without the presence of any density gradient (Eilertsen 1993). Hegseth et al. (1995) observed that spring bloom developed in north Norwegian fjords when the heat flux from water to air became negative and the upper water masses became neutral stable. We assume that such a shift took place between 15 and 18 April, as the sea ice broke up and light conditions together with neutral stable water masses favored phytoplankton growth. We therefore define 18 April as the beginning of the phytoplankton spring bloom in 2002. Water temperature never exceeded 0°C throughout our sampling period and we categorized 2002 as a cold year dominated by Arctic water masses (Table 4). From 10 to 13 May, there was a shift in water masses dominating the sampling station. The salinity dropped, but the temperature stayed in the same range showing increased temperature in the surface water (Fig. 3). Willis et al. (2006) showed that there was an intrusion of Arctic water across the hydrographical front, which separates the shelf water from the WCW inside the fjord, by the end of May 2002. The shift in water masses we observed between 10 and 13 May likely represents the same signal given that their station was placed on the north side of the fjord’s circulation.
Phytoplankton spring bloom dynamics
In 2002, the phytoplankton spring bloom started around 18 April and probably peaked before 1 May, unfortunately in the period when ice conditions prevented sampling. No strong stratification was observed in April and May 2002, but the weak thermal stabilization of the water masses together with the break-up of the sea ice made the growth conditions for phytoplankton favorable. It has previously been observed in Norwegian fjords (Eilertsen 1993; Hegseth et al. 1995) and in the Gulf of Maine (Townsend et al. 1992) that the spring bloom can develop without strong vertical stratification. We assume our sampling on 1 May to have been close to the peak of the bloom since primary production rates were still relatively high, compared to what have been measured during peak blooms in the marginal ice zone in the Barents Sea (Vernet et al. 1998; Hodal and Kristiansen 2008). We therefore categorize this as a peak stage. The spring bloom was dominated by diatoms, as supported by the nutrient profiles with silicate being depleted within the same time frame (April 18 to May 1 2002) as nitrate. This correspond well with the good correlation between Chl-a and biogenic silica together with the taxonomic analysis. Nitrate was slightly depleted in the surface waters at 18 April, indicating that flagellates and non-silicate requiring algae dominated the pre-bloom period. Unfortunately did our analysis not cover the flagellates, leaving our interpretation to be based on indirect measurements like nutrient consumption patterns. Fragilariopsis species dominated the few large cells present on 18 April. Species of this genus are often the first diatom in the succession of phytoplankton spring blooms at higher latitudes (von Quillfeldt 2000) and were also the dominating diatom in the early phase of the spring bloom in late April 2008 (K. Sperre, unpubl.). In May, diatoms such as Thalassiosira spp. and Chaetoceros spp. dominated the phytoplankton community and it was not until mid May that large amounts of P. pouchetii colonies were observed. The observation of P. pouchetii colonies fell together with the intrusion of water masses across the shelf (see above) into the fjord. Since only cells larger than ~20 μm were analyzed in this study, it is not possible to say anything regarding contribution from the smaller cells. But in the marginal ice zone of the northern Barents Sea, the small cells were found to be important also during spring blooms. Small cells (<10 μm) contributed 46% to total primary production (Hodal and Kristiansen 2008).
Even though spring blooms can be ephemeral, we do think that our study together with several additional studies (Table 4) have captured enough information regarding timing, intensity and dominating phytoplankton species to make some overall hypothesises regarding the spring bloom dynamics in Kongsfjorden. From the studies presented in Table 4, we argue that the timing and intensity of the spring bloom in the fjord vary considerably among years. No clear pattern appears between years dominated with Arctic or Atlantic water masses. But in the three “Arctic” years (2002–2004), there were observed differences in stabilization of the water column. In 2003, when the spring bloom was delayed to May no thermal stratification developed before late May (Leu et al. 2006). We therefore hypothesize that meteorological factors have a large impact on the timing of the spring bloom in years, when the fjord is dominated by Arctic water. Regarding the dominating phytoplankton species during the bloom, the pattern seems more distinct between Arctic and Atlantic years. When the fjord is dominated by Arctic water, a hydrographical front at the entrance of the fjord separates the fjord system from the Atlantic water on the shelf (Svendsen et al. 2002; Cottier et al. 2007). Willis et al. (2006) demonstrated that there was a close relationship between water mass advection into Kongsfjorden and changes in zooplankton community structure. It is likely that this can be valid for phytoplankton species as well. We argue that diatoms dominate the spring bloom when Arctic water masses prevail and that either P. pouchetii colonies alone or in combination with diatoms dominate the spring bloom when Atlantic water dominates. Although, also annual differences in the community composition of grazers and available nutrients could have an influence on the succession of phytoplankton species during the spring bloom. Hegseth and Tverberg (2008) have looked at the differences between two Atlantic years (2006 and 2007). They observed that the timing and intensity of the inflow of Atlantic water to the fjord had consequences for the phytoplankton species succession. In 2006, the inflow of Atlantic water to the fjord weakened after February and facilitated the winter convection in March and April, which is an important process to re-suspend resting spores of diatoms to the water column. In 2007, the inflow appeared continuously and inhibited the winter convection resulting in a delay of the spring bloom, which was completely dominated by Phaeocystis (Hegseth and Tverberg 2008). We acknowledge that the studies in Table 4 are only snapshots in a long perspective and longer time series are needed to fully understand the annual variations in the spring bloom dynamics in Kongsfjorden.
Primary production
Only a few primary production measurements have been published from Kongsfjorden (Eilertsen et al. 1989; Hop et al. 2006; Piwosz et al. 2009; Rokkan Iversen and Seuthe 2010) and only one from spring (Rokkan Iversen and Seuthe 2010). Our primary production measurements contribute with a considerable increase in time resolution for this important period of the year. In this study, we did not measure primary production until 1 May after the decline of nutrients (Fig. 4a). The highest integrated primary production measured in this study (1.52–1.85 g C m−2 day−1) was much higher than what Rokkan Iversen and Seuthe (2010) found (0.4 g C m−2 day−1) during the peak of the dense bloom in 2006 (10 mg Chl-a m−3). The integrated primary production from the middle of May 2002 was in the same range as what Rokkan Iversen and Seuthe (2010) found at the end of May 2006. The integrated values lie within the range of earlier measurements from the marginal ice zone in the Barents Sea and from Fram Strait (Smith et al. 1987; Hirche et al. 1991; Vernet et al. 1998; Hodal and Kristiansen 2008). In the first 2 weeks of May, the primary production rates were relatively high even though only moderate concentrations of Chl-a were measured. This resulted in a very high assimilation index (highest 86 mg C [mg Chl-a]−1 day−1). Good growth conditions is assumed based on the assimilation index from integrated values (14–36 mg C (mg Chl-a)−1 day−1) which exceeded what Hodal and Kristiansen (2008) found during blooms in the marginal ice zone (3–10 mg C (mg Chl-a)−1 day−1). In mid May, primary production was highest at 5 or 10 m, probably caused by low biomass at the surface (Table 2). Photoinhibition could also be an explanation but Leu et al. (2006) showed that when the water column was homogenous the phytoplankton cells did not stay in the surface layer long enough to suffer from photodamage. Episodic events of stormy weather and following intrusion of nutrients from deeper water masses might have maintained primary production until 13 May, after which it remained very low. We estimated the total primary production during the spring bloom in 2002 (18 April to 13 May) to range 27–35 g C m−2 depending if integrations were done to 30 or 40 m. The estimate is based on nitrate consumption from 18 April to 30 April in 2002 (18–24 g C m−2) and on measured primary production rates from 1 May to 13 May in 2002 (9–11 g C m−2). To use nitrate consumption to calculate primary production provides a rough estimate, since there can be water exchange and also re-mineralization of nutrients. With the uncertainties in mind, we still think this method can provide a reasonable estimate. If we instead used the measured integrated primary production value from 1 May (1.52–1.85 g C m−2) as an average for the 13 previous days in April, we would get almost the same result (20–24 g C m−2). Due to lack of good depth and time resolution, the assumptions made to calculate total primary production during spring makes the estimate an approximation. Hop et al. (2002) summarized the few data on primary production and estimated that total annual primary production vary between 4 and 180 g C m−2, indicating large temporal variability in Kongsfjorden. Eilertsen et al. (1989) estimated annual primary production to 150 g C m−2. Our estimate of primary production (27–35 g C m−2) during the spring 2002 constituted 0.15–8.75 times the earlier estimates of the total annual primary production in the fjord (Eilertsen et al. 1989; Hop et al. 2002). Indeed the estimations made by Hop et al. (2002) and Eilertsen et al. (1989) are based on few measurements and highlight the need of a better resolution in primary production measurements. Our approach provides better time resolution but still not sufficient to catch the high variability.
Grazers
Dinoflagellates dominated the protozoan community both in abundance and carbon biomass. The abundance of thecate dinoflagellates was low compared to that of athecate dinoflagellates, which fits well with the suggestion by Levinsen and Nielsen (2002) that this is characteristic for Arctic waters. Combined biomass of ciliates and dinoflagellates was lower than what Seuthe et al. (2010) observed during the peak of the bloom in April 2006, but higher than what they found in a post-bloom scenario at the end of May 2006. It is expected that the abundance of protozoans will be lower in a year with less accumulation of phytoplankton since the growth rate of heterotrophic protozoans is heavily reduced when food availability (Chl-a) is reduced (Sherr and Sherr 2007). The samples in our study were also stored longer than what is optimal and some material may have degraded resulting in an underestimate of protozoan biomass. A fraction of the protozoans might be mixotrophic as shown by Seuthe et al. (2010) in April 2006 while another part may be grazing on the phytoplankton and the protozoan them self. Grazing on protozoans from mesozooplankton was thought to be very low due to the very low abundance of mesozooplankton in spring (Walkusz et al. 2009).
Walkusz et al. (2009) investigated the zooplankton community in Kongsfjorden in the middle of April 2002 and found that the small zooplankton Oithona similis dominated, with as low abundance as 348 ind. m−3 at the same sampling station as in our study on 15 April. Calanus finmarchicus was barely present in the surface layer in the fjord (<13 ind. m−3). The same authors also found Cirripedia larvae in spring but only at two stations in the outer part of the fjord. They were observed to be very patchy distributed with mass appearances of 3,870 ind. m−3. After 13 May, we also observed Cirripedia larvae, coincident with the advection of water masses from the shelf area. This sudden appearance could also be caused by local populations and the fact that they are released in pulses. Mass appearances of Cirripedia larvae have also been observed in Kongsfjorden in 1989 (Eilertsen et al. 1989) and in 2002 (Willis et al. 2006), in Isfjorden (Zajaczkowski et al. 2010) and in Hornsund (Piwosz et al. 2009). High abundances in Kongsfjorden could be explained by the presence of large areas of hard bottom in the near shore areas of the middle and the outer parts of the fjord (Jørgensen and Gulliksen 2001; Kaczmarek et al. 2005). To illustrate how high a grazing potential this group can have at such high abundances, we have used the literature values on abundance (Walkusz et al. 2009) and ingestion rate (Pasternak et al. 2008) and calculated a potential consumption rate of 52 mg C m−3 day−1 from this group.
Fate of the spring bloom
During the first part of May, we measured relatively high primary production rates, but the concentrations of Chl-a were moderate. Removal processes must therefore have been equal or higher than production. Grazing and sedimentation are in principle two competing processes. Less algal biomass is exported during times of intensive grazing, whereas more is exported when grazing is low (Reigstad et al. 2000; Sakshaug et al. 2009). The mesozooplankton abundance was found to be very low (Walkusz et al. 2009), and we consider grazing from this group to be of minor importance. The protozoans may partly be grazing on the phytoplankton cells but some diatoms have developed defense mechanisms to prevent protozoan grazers. Also, a fraction of the protozoans might be mixotrophic and this leads to the theory that protozoans might not have grazed the bulk of the diatom-dominated spring bloom.
By the end of May, we observed higher concentrations of Chl-a at 60 m than at the surface. The 1% light depth seems to be 30–40 m in Kongsfjorden during May (S. Kristiansen, unpubl. data). It is unlikely that the biomass was from deep primary production but rather is a result of sinking phytoplankton. Also in the years 2003, 2006 and 2007, the biomass of phytoplankton was assumed to be vertically exported (Leu et al. 2006; Hegseth and Tverberg 2008; Narcy et al. 2009). Based on these conclusions (high primary production, only moderate grazing and observed sinking biomass), we hypothesize that a large part of the primary production during the peak of the bloom is vertically exported. This is in contrast to what Wiktor (1999) concluded. He measured a low sedimentation rate of 1.4 mg C m−2 day−1 during spring in Kongsfjorden. There is no information on the grazing pressure from mesozooplankton or protozoans in that work and it is difficult to conclude if the low vertical export that year was caused by a heavy grazing pressure. Since there seem to be a mismatch between primary production and mesozooplankton during spring in Kongsfjorden in 2002, the protozoan community likely plays an important ecological role in transferring the carbon to higher trophic levels. This is also supported by Rokkan Iversen and Seuthe (2010) who found that the microbial food web in Kongsfjorden was in a “transfer mode” at this time of year. There is a need for more detailed system–ecological studies to fully reveal the fate of the primary production in Kongsfjorden, with simultaneous investigations of pelagic processes, biomass developments and vertical export.
Conclusion
The development of the diatom-dominated spring bloom was initiated by the break-up of sea ice and of stabilized water masses. The present study demonstrates that the phytoplankton spring bloom in Kongsfjorden in 2002 started around 18 April and lasted until middle of May (13 May) after which the bloom went into a post-bloom stage, characterized by low nutrient concentrations, low primary production and phytoplankton biomass. Our data have a higher temporal resolution than previously published studies and we estimated the primary production during the spring, 18 April–13 May of 2002 to be 27–35 g C m−2. Low accumulation of biomass was observed in beginning of May even though high primary production rates were measured. Given the assumed moderate grazing potential for the spring bloom period, we hypothesize that a large fraction of primary production during the spring bloom was vertically exported.
References
Basedow SL, Eiane K, Tverberg V, Spindler M (2004) Advection of zooplankton in an Arctic fjord (Kongsfjorden, Svalbard). Estuar Coast Shelf Sci 60:113–124. doi:10.1016/j.ecss.2003.12.004
Cottier F, Tverberg V, Inall M, Svendsen H, Nilsen F, Griffiths C (2005) Water mass modification in an Arctic fjord through cross-shelf exchange: the seasonal hydrography of Kongsfjorden, Svalbard. J Geophys Res Oceans 110:1–18. doi:10.1029/2004jc002757
Cottier FR, Nilsen F, Inall ME, Gerland S, Tverberg V, Svendsen H (2007) Wintertime warming of an Arctic shelf in response to large-scale atmospheric circulation. Geophys Res Lett 34. doi:10.1029/2007gl029948
Cushing DH (1992) The loss of diatoms in the spring bloom. Philos Trans R Soc Lond Ser B Biol Sci 335:237–246
Degerlund M, Eilertsen HC (2010) Main species characteristics of phytoplankton spring blooms in NE Atlantic and Arctic waters (68–80°N). Estuar Coasts 33:242–269. doi:10.1007/s12237-009-9167-7
Eilertsen HC (1993) Spring blooms and stratification. Nature 363:24
Eilertsen HC, Schei B, Taasen JP (1981) Investigations on the plankton community of Balsfjorden, northern Norway—the phytoplankton 1976–1978—abundance, species composition, and succession. Sarsia 66:129–141
Eilertsen HC, Taasen JP, Weslawski JM (1989) Phytoplankton studies in the fjords of west Spitzbergen—physical-environment and production in spring and summer. J Plankton Res 11:1245–1260
Gargas E (1975) A manual for phytoplankton primary production studies in the Baltic. The Danish Agency of Environmental Protection, Hørsholm
Hegseth EN, Tverberg V (2008) Changed spring bloom timing in a Svalbard (high Arctic) fjord caused by Atlantic water inflow? Paper presented at the SCAR conference ‘polar research-arctic and antarctic perspectives in the international polar year’, St. Petersburg, 7–11 July 2008
Hegseth EN, Svendsen H, von Quillfeldt CH (1995) Phytoplankton in fjords and coastal waters of northern Norway: environmental conditions and dynamics of the spring bloom. Ecology of Fjords and Coastal Waters. Elsevier Science Publ B.V., Amsterdam
Hirche HJ, Baumann MEM, Kattner G, Gradinger R (1991) Plankton distribution and the impact of copepod grazing on primary production in Fram Strait, Greenland Sea. J Mar Syst 2:477–494
Hodal H, Kristiansen S (2008) The importance of small-celled phytoplankton in spring blooms at the marginal ice zone in the northern Barents Sea. Deep Sea Res II 55:2176–2185. doi:10.1016/j.dsr2.2008.05.012
Hop H, Pearson T, Hegseth EN, Kovacs KM, Wiencke C, Kwasniewski S, Eiane K, Mehlum F, Gulliksen B, Wlodarska-Kowalzuk M, Lydersen C, Weslawski JM, Cochrane S, Gabrielsen GW, Leakey RJG, Lønne OJ, Zajaczkowski M, Falk-Petersen S, Kendall M, Wängberg SÅ, Bischof K, Voronkov AY, Kovaltchouk NA, Wiktor J, Poltermann M, di Prisco G, Papucci C, Gerland S (2002) The marine ecosystem of Kongsfjorden, Svalbard. Polar Res 21:167–208
Hop H, Falk-Petersen S, Svendsen H, Kwasniewski S, Pavlov V, Pavlova O, Søreide JE (2006) Physical and biological characteristics of the pelagic system across Fram Strait to Kongsfjorden. Prog Oceanogr 71:182–231. doi:10.1016/j.pocean.2006.09.007
Janowska K, Wlodarska-Kowalzuk M, Wieczorek P (2005) Abundance and biomass of bacteria in two glacial fjords. Pol Polar Res 26:77–84
Jørgensen LL, Gulliksen B (2001) Rocky bottom fauna in arctic Kongsfjord (Svalbard) studied by means of suction sampling and photography. Polar Biol 24:113–121
Kaczmarek H, Wlodarska-Kowalzuk M, Legezynska J, Zajaczkowski M (2005) Shallow sublitoral macrozoobenthos in Kongsfjorden, west Spitsbergen, Svalbard. Pol Polar Res 26:137–155
Leu E, Falk-Petersen S, Kwasniewski S, Wulff A, Edvardsen K, Hessen DO (2006) Fatty acid dynamics during the spring bloom in a High Arctic fjord: importance of abiotic factors versus community changes. Can J Fish Aquat Sci 63:2760–2779. doi:10.1139/f06-159
Levinsen H, Nielsen TG (2002) The trophic role of marine pelagic ciliates and heterotrophic dinoflagellates in arctic and temperate coastal ecosystems: a cross-latitude comparison. Limnol Oceanogr 47:427–439
Lischka S, Hagen W (2005) Life histories of the copepods Pseudocalanus minutus, P. acuspes (Calanoida) and Oithona similis (Cyclopoida) in the Arctic Kongsfjorden (Svalbard). Polar Biol 28:910–921. doi:10.1007/s00300-005-0017-1
Lischka S, Hagen W (2007) Seasonal lipid dynamics of the copepods Pseudocalanus minutus (Calanoida) and Oithona similis (Cyclopoida) in the Arctic Kongsfjorden (Svalbard). Mar Biol 150:443–454. doi:10.1007/s00227-006-0359-4
Lischka S, Gimenez L, Hagen W, Ueberschar B (2007) Seasonal changes in digestive enzyme (trypsin) activity of the copepods Pseudocalanus minutus (Calanoida) and Oithona similis (Cyclopoida) in the Arctic Kongsfjorden (Svalbard). Polar Biol 30:1331–1341. doi:10.1007/s00300-007-0294-y
Lovejoy C, Vincent WF, Bonilla S, Roy S, Martineau MJ, Terrado R, Potvin M, Massana R, Pedros-Alio C (2007) Distribution, phylogeny, and growth of cold-adapted picoprasinophytes in arctic seas. J Phycol 43:78–89. doi:10.1111/j.1529-8817.2006.00310.x
Menden-Deuer S, Lessard EJ (2000) Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol Oceanogr 45:569–579
Narcy F, Gasparini S, Falk-Petersen S, Mayzaud P (2009) Seasonal and individual variability of lipid reserves in Oithona similis (Cyclopoida) in an Arctic fjord. Polar Biol 32:233–242. doi:10.1007/s00300-008-0524-y
Okolodkov YB, Hapter R, Semovski SV (2000) Phytoplankton in Kongsfjorden, Spitsbergen, July 1996. Sarsia 85:345–352
Paasche E (1980) Silicon content of 5 marine plankton diatom species measured with a rapid filter method. Limnol Oceanogr 25:474–480
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, Oxford
Pasternak A, Arashkevich E, Reigstad M, Wassmann P, Falk-Petersen S (2008) Dividing mesozooplankton into upper and lower size groups: applications to the grazing impact in the Marginal Ice Zone of the Barents Sea. Deep Sea Res II 55:2245–2256. doi:10.1016/j.dsr2.2008.05.002
Piquet AM-T, Scheepens JF, Bolhus H, Wiencke C, Buma AGJ (2010) Variability of protistian and bacterial communities in two Arctic fjords (Spitsbergen). Polar Biol 33:1521–1536. doi:10.1007/s00300-010-0841-9
Piwosz K, Walkusz W, Hapter R, Wieczorek P, Hop H, Wiktor J (2009) Comparison of productivity and phytoplankton in a warm (Kongsfjorden) and a cold (Hornsund) Spitsbergen fjord in mid-summer 2002. Polar Biol 32:549–559. doi:10.1007/s00300-008-0549-2
Redfield A (1934) On the proportions of organic derivates in sea water and their relation to the composition of plankton. James Johnstone Memorial volume, Liverpool, pp 176–192
Reigstad M, Wassmann P, Ratkova T, Arashkevich E, Pasternak A, Øygarden S (2000) Comparison of the springtime vertical export of biogenic matter in three northern Norwegian fjords. Mar Ecol Prog Ser 201:73–89
Rokkan Iversen K, Seuthe L (2010) Seasonal microbial processes in a high-latitude fjord (Kongsfjorden, Svalbard): I. Heterotrophic bacteria, picoplankton and nanoflagellates. Polar Biol. doi:10.1007/s00300-010-0929-2
Sakshaug E (2004) Primary and secondary production in the Arctic Seas. In: Stein R, Macdonald RW (eds) The organic carbon cycle in the Arctic Ocean. Springer, Berlin, pp 57–81
Sakshaug E, Johnsen G, Kristiansen S, von Quillfeldt CH, Rey F, Slagstad D, Thingstad F (2009) Phytoplankton and primary production. In: Sakshaug E, Johnsen G, Kovacs KM (eds) Ecosystem Barents Sea. Tapir Academic Press, Tronheim, pp 167–208
Seuthe L, Rokkan Iversen K, Narcy F (2010) Microbial processes in a high-latitude fjord (Kongsfjorden, Svalbard): II. Ciliates and dinoflagellates. Polar Biol. doi:10.1007/s00300-010-0930-9
Sherr EB, Sherr BF (2007) Heterotrophic dinoflagellates: a significant component of microzooplankton biomass and major grazers of diatoms in the sea. Mar Ecol Prog Ser 352:187–197. doi:10.3354/meps07161
Smetacek V, Nicol S (2005) Polar ocean ecosystems in a changing world. Nature 437:362–368. doi:10.1038/nature04161
Smith WO, Baumann MEM, Wilson DL, Aletsee L (1987) Phytoplankton biomass and productivity in the marginal ice-zone of the Fram Strait during summer 1984. J Geophys Res Oceans 92:6777–6786
Strickland JDH, Parsons TR (1972) A practical handbook of seawater analysis, 2nd edn. Fisheries Research Board of Canada 167, Ottawa
Svendsen H, Beszczynska-Møller A, Hagen JO, Lefauconnier B, Tverberg V, Gerland S, Ørbæk JB, Bischof K, Papucci C, Zajaczkowski M, Azzolini R, Bruland O, Wiencke C, Winther J-G, Dallmann W (2002) The physical environment of Kongsfjorden-Krossfjorden, an Arctic fjord system in Svalbard. Polar Res 21:133–166
Townsend DW, Keller MD, Sieracki ME, Ackleson SG (1992) Spring phytoplankton blooms in the absence of vertical water column stratification. Nature 360:59–62
Vernet M, Matrai PA, Andreassen I (1998) Synthesis of particulate and extracellular carbon by phytoplankton at the marginal ice zone in the Barents Sea. J Geophys Res Oceans 103:1023–1037
von Quillfeldt CH (2000) Common diatom species in Arctic spring blooms: their distribution and abundance. Bot Mar 43:499–516
Walczowski W, Piechura J (2006) New evidence of warming propagating toward the Arctic Ocean. Geophys Res Lett 33. doi:10.1029/2006gl025872
Walczowski W, Piechura J (2007) Pathways of the Greenland Sea warming. Geophys Res Lett 34. doi:10.1029/2007gl029974
Walkusz W, Kwasniewski S, Falk-Petersen S, Hop H, Tverberg V, Wieczorek P, Weslawski JM (2009) Seasonal and spatial changes in the zooplankton community of Kongsfjorden, Svalbard. Polar Res 28:254–281. doi:10.1111/j.1751-8369.2009.00107.x
Wang GZ, Guo CY, Luo W, Cai MH, He JF (2009) The distribution of picoplankton and nanoplankton in Kongsfjorden, Svalbard during late summer 2006. Polar Biol 32:1233–1238. doi:10.1007/s00300-009-0666-6
Wassmann P (1998) Retention versus export food chains: processes controlling sinking loss from marine pelagic systems. Hydrobiologia 363:29–57
Wassmann P, Ratkova T, Andreassen I, Vernet M, Pedersen C, Rey F (1999) Spring bloom development in the marginal ice zone and the central Barents Sea. Mar Ecol Publ Stn Zoo Napoli 20:321–346
Wiktor J (1999) Early spring microplankton development under fast ice covered fjords of Svalbard, Arctic. Oceanologia 41:51–72
Wiktor J, Wojciechowska K (2005) Differences in taxonomic composition of summer phytoplankton in two fjords of West Spitsbergen, Svalbard. Pol Polar Res 26:259–268
Willis K, Cottier F, Kwasniewski S, Wold A, Falk-Petersen S (2006) The influence of advection on zooplankton community composition in an Arctic fjord (Kongsfjorden, Svalbard). J Mar Syst 61:39–54. doi:10.1016/j.jmarsys.2005.11.013
Zajaczkowski M, Nygård H, Hegseth EN, Berge J (2010) Vertical flux of particulate matter in an Arctic fjord: the case of lack of the sea-ice cover in Adventfjorden 2006–2007. Polar Biol 33:223–239. doi:10.1007/s00300-009-0699-x
Acknowledgments
The authors wish to thank Marte Lundberg and employees at the Norwegian Polar institute in Ny-Ålesund for great assistance during the difficult conditions in April and May 2002. We also thank Eva Leu, Sten-Åke Wängberg and two anonymous reviewers for their constructive comments, which improved the manuscript. The investigation was conducted with financial support from Arktisk Stipend, Norwegian Polar Institute, and a grant from Amundsen Center of the University of Tromsø.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Hodal, H., Falk-Petersen, S., Hop, H. et al. Spring bloom dynamics in Kongsfjorden, Svalbard: nutrients, phytoplankton, protozoans and primary production. Polar Biol 35, 191–203 (2012). https://doi.org/10.1007/s00300-011-1053-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-011-1053-7