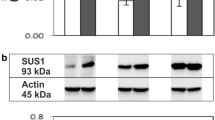
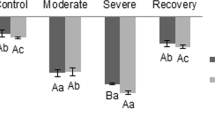
Abstract
Colobanthus quitensis (Kunth) Bartl. is widely distributed from Mexico to the Antarctic. C. quitensis is a freezing resistant species that accumulates sucrose in response to cold. We tested the hypothesis that low temperature modifies the kinetic properties of C. quitensis sucrose phosphate synthase (SPS) to increase its activity and ability to synthesize sucrose during cold acclimation. Cold acclimation caused a fourfold increment in sucrose concentration and a 100% increase in SPS activity, without changes in the level of SPS protein. Cold acclimation did not affect the optimal temperature and pH for SPS activity. However, it caused a tenfold increase in the inhibition constant (K i) for inorganic phosphate (Pi) calculated as a function of fructose-6-phosphate (Fruc-6-P). SPS from cold acclimated plants also exhibited a higher reduction of its Michaelis constant (K m) for glucose-6-phosphate (Gluc-6-P) with respect to non-acclimated plants. We suggest that the increase in C. quitensis SPS K i for Pi and the increase in activation by Gluc-6-P in response to cold keep SPS activated, leading to high sucrose accumulation. This may be an important adaptation that allows efficient accumulation of sucrose during the harsh Antarctic summer.





Similar content being viewed by others
References
Alberdi M, Bravo LA, Gutiérrez A, Gidekel M, Corcuera LJ (2002) Ecophysiology of Antarctic vascular plants. Physiol Plant 115:479–486
Amir J, Preiss J (1982) Kinetic characterization of spinach leaf sucrose phosphate. Plant Physiol 69:1027–1030
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bravo LA, Ulloa N, Zuñiga GE, Casanova A, Corcuera LJ, Alberdi M (2001) Cold resistance in Antarctic angiosperms. Physiol Plant 111:55–65
Deiting U, Zrenner R, Stitt M (1998) Similar temperature requirement for sugar accumulation and for the induction of new forms of sucrose phosphate synthase and amylase in cold-stored potato tubers. Plant Cell Environ 21:127–138
Gianoli E, Inostroza P, Zúñiga-Feest A, Reyes-Díaz M, Cavieres LA, Bravo LA, Corcuera LJ (2004) Ecotypic differentiation in morphology and cold resistance in populations of Colobanthus quitensis (Cariophyllaceae) from the Andes of Central Chile and Maritime Antarctica. Arctic Antarctic and Alpine Research 36:484–489
Guy CL, Huber JL, Huber SC (1992) Sucrose phosphate synthase and sucrose accumulation at low temperature. Plant Physiol 100:502–503
Hauch S, Magel E (1998) Extractable activities and protein content of sucrose-phosphate synthase, sucrose synthase and neutral invertase in trunk tissues of Robinia pseudoacacia are related to cambial wood production and heartwood formation. Planta 207:266–274
Huber SC, Huber JL (1996) Role and regulation of sucrose-phosphate synthase in higher plants. Annu Rev Plant Physiol Plant Mol Biol 17:431–444
Hurry V, Strand A, Furbank R, Stitt M (2000) The role of inorganic phosphate in the development of freezing tolerance and the acclimatization of photosynthesis to low temperature is revealed by the pho mutants of Arabidopsis thaliana. Plant J 24:383–396
Laemli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lee KY, Nurok D, Zlatkis A (1979) Determination of glucose, fructose and sucrose in molasses by high-performance thin-layer chromatography. J Chromatogr 174:187–193
Lewis-Smith RI, Poncet S (1987) Deschampsia antarctica and Colobanthus quitensis in the Terra Firma Islands. Br Antarct Surv Bull 74:31–35
Lewis-Smith RI (2003) The enigma of Colobanthus quitensis and Deschampsia antarctica in Antyarctica. In: Huiskes AHL, Gieskes WWC, Rozema J, Schorno RML, van der vies SM, Wolff WJ (eds) Antarctic biology in a global context. Backhuys Publishers, Leiden, pp 234–239
Niittyla T, Messerli G, Trevisan M, Chen J, Smith AM, Zeeman SC (2004) A previously unknown maltose transporter essential for starch degradation in leaves. Science 303:87–89
Perez P, Morcuende R, Martin Del Molino I, Sanchez De La Puente L, Martinez-Carrasco R (2001) Contrasting responses of photosynthesis and carbon metabolism to low temperatures in tall fescue and clovers. Physiol Plant 112:478–486
Pérez-Torres E, Dinamarca J, Bravo LA, Corcuera LJ (2004) Responses of Colobanthus quitensis (Kunth) Bartl. to high light and low temperature. Polar Biol 27:183–189
Reimholz R, Geiger M, Deiting U, Krause KP, Sonnewald U, Stitt M (1997) Potato plants contain multiple forms of sucrose phosphate synthase, that show differences in their tissue distribution, their response during development, and their response to low temperature. Plant Cell Environ 20:291–305
Santarius KA (1992) Freezing of isolated thylakoid membranes in complex media VIII Differential cryoprotection by sucrose, proline and glycerol. Physiol Plant 84:87–93
Sinha AK, Pathre UV, Sane PV (1997) Purification and characterization of sucrose-phosphate synthase from Prosopis juliflora. Phytochemistry 46(3):441–447
Stitt M, Kürzel B, Heldt HW (1984) Control of photosynthetic sucrose synthesis by fructose 2,6-bisphosphate II partitioning between sucrose and starch. Plant Physiol 75:554–560
Theodorou ME, Plaxto WC (1993) Metabolic adaptations of plant respiration to nutritional phosphate deprivation. Plant Physiol 101:339–344
Toroser D, Huber SC (1997) Protein phosphorylation as a mechanism for osmotic-stress activation of sucrose-phosphate synthase in spinach leaves. Plant Physiol 114:947–55
Uemura M, Steponkus PL (2003) Modification of the intracellular sugar content alters the incidence of freeze-induced membrane lesions of protoplasts isolated from Arabidopsis thaliana leaves. Plant Cell Environ 26:1083–1096
Xiong FS, Ruhland CT, Day TA (1999) Photosynthetic temperature response of the Antarctic vascular plants Colobanthus quitensis and Deschampsia antarctica. Physiol Plant 106:276–286
Zrenner R, Stitt M (1991) Comparison of the effect of rapidly and gradually developing water-stress on carbohydrate metabolism in spinach leaves. Plant Cell Environ 14:939–946
Zúñiga-Feest A, Ort D, Gutiérrez A, Gidekel M, Bravo LA, Corcuera LJ (2005) Light regulation of sucrose-phosphate synthase activity in the freezing-tolerant grass Deschampsia antarctica. Photosynth Res 83:75–86
Acknowledgements
Permits to collect plant material in protected areas in the Maritime Antarctic were provided by Instituto Antártico Chileno. The authors thank the financial support of DIUC 205.111.042-1S. Luisa Bascuñán thanks MECESUP UCO 0214 for a graduate fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bascuñán-Godoy, L., Uribe, E., Zúñiga-Feest, A. et al. Low temperature regulates sucrose-phosphate synthase activity in Colobanthus quitensis (Kunth) Bartl. by decreasing its sensitivity to Pi and increased activation by glucose-6-phosphate. Polar Biol 29, 1011–1017 (2006). https://doi.org/10.1007/s00300-006-0144-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-006-0144-3