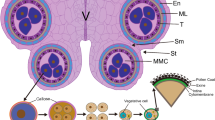
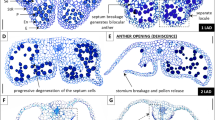
Abstract
Key message
The ‘neglected’ thermophile fruit crop of watermelon was first used as a model crop to study the PCD associated with anther dehiscence in cold-exposed condition during anther development.
Abstract
Anther dehiscence ensures normal pollen release and successful fertilization at fruit-setting stages in flowering plants. However, most researches pertinent to anther dehiscence are centered on model plant and/or major field crops under optimal growth condition. Due to anther indehiscence in cold condition, crop plants of thermophile tropical or subtropical fruit crops fail to accomplish timely pollination and fertilization, resulting in a great yield loss annually. Herein, we developed an ideal model crop for studying the programmed cell death (PCD) associated with anther dehiscence under low-temperature stress using the S-shaped spiral anther in watermelon as instead. Our results revealed that, including the tapetal cell layers, both cells of the interlocular septum and the stomium were blocked in PCD associated with anther dehiscence at 15 °C. Likewise, TUNEL assays visualized the evidence that low temperature at 15 °C interferes with not only the PCD of tapetal cells, but also the PCD of interlocular septum and stomium. Furthermore, the expressions of genes correlated with PCD of tapetum and stomium were significantly inhibited at 15 °C, suggesting that low temperature affects anther dehiscence by inhibiting PCD of sporophytic tissue-related gene expressions. The findings of the current research provide mechanistic insights into anther indehiscence leading to poor fruit-setting for thermophile fruit crop such as watermelon under adverse cold condition in flowering.





Similar content being viewed by others
References
Alexander MP (1969) Differential staining of aborted and non-aborted pollen. Stain Technol 44(3):117–122
Atluri JB, Ramana SV, Reddi CS (2004) Explosive pollen release, wind-pollination and mixed mating in the tropical tree Shorea robusta Gaertn. f. (Dipterocarpaceae). Curr Sci 86(10):1416–1419
Barton DA, Cantrill LC, Law AMK, Phillips CG, Sutton BG, Overall RL (2014) Chilling to zero degrees disrupts pollen formation but not meiotic microtubule arrays in Triticum aestivum L. Plant Cell Environ 37(12):2781–2794
Bonner LJ, Dickinson HG (1990) Anther dehiscence in Lycopersicon esculentum: II. Water relations. New Phytol 115(2):367–375
Chen KY, Cong B, Wing R, Vrebalov J, Tanksley SD (2007) Changes in regulation of a transcription factor lead to autogamy in cultivated tomatoes. Science 318(5850):643–645
Dai SY, Hsu WH, Yang CH (2019) The gene ANTHER DEHISCENCE REPRESSOR (ADR) controls male fertility by suppressing the ROS accumulation and anther cell wall thickening in Arabidopsis. Sci Rep 9(1):5112
Davis GL (1966) Systematic embryology of the angiosperms. Wiley, New York, p 528
Dawson J, Sözen E, Vizir I, Van Waeyenberge S, Wilson ZA, Mulligan BJ (1999) Characterization and genetic mapping of a mutation (ms35) which prevents anther dehiscence in Arabidopsis thaliana by affecting secondary wall thickening in the endothecium. New Phytol 144(2):213–222
Fu BX, Bellis GA, Hong J, Wang JR, Wu Q, Tang QY, Cheng JA, Zhu ZR (2012) Morphology, distribution, and abundance of antennal sensilla of male and female macropterous and brachypterous small brown planthopper, Laodelphax striatellus (Fallén) (Hemiptera: Delphacidae). Microsc Res Tech 75(11):1492–1512
García CC (2002) An approach to the diversity of endothecial thickenings in Solanaceae. Flora 197(3):214
Gorczyca W, Bruno S, Darzynkiewicz R, Gong J, Darzynkiewicz Z (1992) DNA strand breaks occurring during apoptosis—their early in situ detection by the terminal deoxynucleotidyl transferase and nick translation assays and prevention by serine protease inhibitors. Int J Oncol 1(6):639–648
Hou W, Yang F, Li S, Zhou Z, Chen H, Wu C (2015) Effects of low temperature and low light on the growth of watermelon grown in greenhouse in Hainan and their disaster grade indexes. Jiangsu Agric Sci 43(8):161–166
Kawanabe T, Ariizumi T, Kawai-Yamada M, Uchimiya H, Toriyama K (2006) Abolition of the tapetum suicide program ruins microsporogenesis. Plant Cell Physiol 47(6):784–787
Keijzer CJ (1987) The processes of anther dehiscence and pollen dispersal. New Phytol 105(3):487–498
King MJ, Buchmann SL (1995) Bumble bee-initiated vibration release mechanism of rhododendron pollen. Am J Bot 82(11):1407–1411
Kong Q, Yuan J, Gao L, Zhao S, Jiang W, Huang Y, Bie Z (2014) Identification of suitable reference genes for gene expression normalization in qRT-RCR analysis in watermelon. PLOS One 9(2):e90612
Ku S, Yoon H, Suh HS, Chung YY (2003) Male-sterility of thermosensitive genic male-sterile rice is associated with premature programmed cell death of the tapetum. Planta 217(4):559–565
Li N, Zhang D, Liu H, Yin C, Li X, Liang W, Yuan Z, Xu B, Chu H, Wang J, Wen T (2006) The rice tapetum degeneration retardation gene is required for tapetum degradation and anther development. Plant Cell 18(11):2999–3014
Li H, Yuan Z, Vizcay-Barrena G, Yang C, Liang W, Zong J, Wilson ZA, Zhang D (2011a) PERSISTENT TAPETAL CELL1 encodes a PHD-finger protein that is required for tapetal cell death and pollen development in rice. Plant Physiol 156(2):615–630
Li X, Gao X, Wei Y, Deng L, Ouyang Y, Chen G, Li X, Zhang Q, Wu C (2011b) Rice APOPTOSIS INHIBITOR5 coupled with two DEAD-box adenosine 5′-triphosphate-dependent RNA helicases regulates tapetum degeneration. Plant Cell 23(4):1416–1434
Li DD, Xue JS, Zhu J, Yang ZN (2017) Gene regulatory network for tapetum development in Arabidopsis thaliana. Front Plant Sci 8:1559
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Millar AA, Gubler F (2005) The Arabidopsis GAMYB-like genes, MYB33 and MYB65, are microRNA-regulated genes that redundantly facilitate anther development. Plant Cell 17(3):705–721
Mitsuda N, Seki M, Shinozaki K, Ohme-Takagi M (2005) The NAC transcription factors NST1 and NST2 of Arabidopsis regulate secondary wall thickenings and are required for anther dehiscence. Plant Cell 17(11):2993–3006
Niu N, Liang W, Yang X, Jin W, Wilson ZA, Hu J, Zhang D (2013) EAT1 promotes tapetal cell death by regulating aspartic proteases during male reproductive development in rice. Nat Commun 4:1445
Oda S, Kaneko F, Yano K, Fujioka T, Masuko H, Park JI, Kikuchi S, Hamada K, Endo M, Nagano K, Nagamura Y (2010) Morphological and gene expression analysis under cool temperature conditions in rice anther development. Genes Genet Syst 85(2):107–120
Oliver SN, Van Dongen JT, Alfred SC, Mamun EA, Zhao X, Saini HS, Fernandes SF, Blanchard CL, Sutton BG, Geigenberger P, Dennis ES (2005) Cold-induced repression of the rice anther-specific cell wall invertase gene OSINV4, is correlated with sucrose accumulation and pollen sterility. Plant Cell Environ 28(12):1534–1551
Parish RW, Phan HA, Iacuone S, Li SF (2012) Tapetal development and abiotic stress: a centre of vulnerability. Funct Plant Biol 39(7):553–559
Phan HA, Iacuone S, Li SF, Parish RW (2011) The MYB80 transcription factor is required for pollen development and the regulation of tapetal programmed cell death in Arabidopsis thaliana. Plant Cell 23(6):2209–2224
Raghavan V (1988) Anther and pollen development in rice (Oryza sativa). Am J Bot 75(2):183–196
Rivero RM, Ruiz JM, Garcıa PC, Lopez-Lefebre LR, Sánchez E, Romero L (2001) Resistance to cold and heat stress: accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci 160(2):315–321
Sanders PM, Bui AQ, Weterings K, McIntire KN, Hsu YC, Lee PY, Truong MT, Beals TP, Goldberg RB (1999) Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex Plant Reprod 11(6):297–322
Sanders PM, Bui AQ, Le BH, Goldberg RB (2005) Differentiation and degeneration of cells that play a major role in tobacco anther dehiscence. Sex Plant Reprod 17(5):219–241
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671
Scott RJ, Spielman M, Dickinson HG (2004) Stamen structure and function. Plant Cell 16(suppl 1):S46–S60
Senatore A, Trobacher CP, Greenwood JS (2009) Ricinosomes predict programmed cell death leading to anther dehiscence in tomato. Plant Physiol 149(2):775–790
Sharma KD, Nayyar H (2016) Regulatory networks in pollen development under cold stress. Front Plant Sci 7:402
Shi P, Guy KM, Wu W, Fang B, Yang J, Zhang M, Hu Z (2016) Genome-wide identification and expression analysis of the ClTCP transcription factors in Citrullus lanatus. BMC Plant Biol 16(1):85
Steiner-Lange S, Unte US, Eckstein L, Yang C, Wilson ZA, Schmelzer E, Dekker K, Saedler H (2003) Disruption of Arabidopsis thaliana MYB26 results in male sterility due to non-dehiscent anthers. Plant J 34(4):519–528
Susanti Z, Snell P, Fukai S, Mitchell JH (2019) Importance of anther dehiscence for low-temperature tolerance in rice at the young microspore and flowering stages. Crop Pasture Sci 70(2):113–120
Thakur P, Kumar S, Malik JA, Berger JD, Nayyar H (2010) Cold stress effects on reproductive development in grain crops: an overview. Environ Exp Bot 67(3):429–443
van Rensburg HJ, Robbertse PJ, Small JGC (1985) Morphology of the anther, microsporogenesis and pollen structure of Momordica balsamina. S Afr J Bot 51(2):125–132
Varnier AL, Mazeyrat-Gourbeyre F, Sangwan RS, Clément C (2005) Programmed cell death progressively models the development of anther sporophytic tissues from the tapetum and is triggered in pollen grains during maturation. J Struct Biol 152(2):118–128
Wei M, Li W, Gao X, Wang X, Zhang X, Zhang Y, Hang X (2015) Low temperature is one of the most serious abiotic stresses and one limiting factor causing great yield loss. Chin J Trop Crops 36(4):821–828
Wei D, Liu M, Chen H et al (2018) INDUCER OF CBF EXPRESSION 1 is a male fertility regulator impacting anther dehydration in Arabidopsis. PLoS Genet 14(10):e1007695
Wilson ZA, Morroll SM, Dawson J, Swarup R, Tighe PJ (2001) The Arabidopsis MALE STERILITY1 (MS1) gene is a transcriptional regulator of male gametogenesis, with homology to the PHD-finger family of transcription factors. Plant J 28(1):27–39
Xu J, Yang C, Yuan Z, Zhang D, Gondwe MY, Ding Z, Liang W, Zhang D, Wilson ZA (2010) The ABORTED MICROSPORES regulatory network is required for postmeiotic male reproductive development in Arabidopsis thaliana. Plant Cell 22(1):91–107
Yi J, Moon S, Lee YS, Zhu L, Liang W, Zhang D, Jung KH, An G (2016) Defective Tapetum Cell Death 1 (DTC1) regulates ROS levels by binding to metallothionein during tapetum degeneration. Plant Physiol 170(3):1611–1623
Zhang W, Sun Y, Timofejeva L, Chen C, Grossniklaus U, Ma H (2006) Regulation of Arabidopsis tapetum development and function by DYSFUNCTIONAL TAPETUM1 (DYT1) encoding a putative bHLH transcription factor. Development 133(16):3085–3095
Zhu J, Chen H, Li H, Gao J, Jiang H, Wang C, Guan Y, Yang Z (2008) Defective in Tapetal development and function 1 is essential for anther development and tapetal function for microspore maturation in Arabidopsis. Plant J 55(2):266–277
Zou C, Jiang W, Yu D (2010) Male gametophyte-specific WRKY34 transcription factor mediates cold sensitivity of mature pollen in Arabidopsis. J Exp Bot 61(14):3901–3914
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 31672175), the Key Science and Technology Program for Agricultural (Vegetable) New Variety Breeding of Zhejiang Province (2016C02051-4-1) and the Earmarked Fund for Modern Agro-Industry Technology Research System of China (CARS-26-17).
Author information
Authors and Affiliations
Contributions
XL, ZH, and MZ conceived and designed the study. XL, SC and NL performed the experiments. XL, JY and JL analyzed the data, and XL and MZ wrote the paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. S1. Mature anther size at 26
°C and 15 °C. (a-d) Schematic diagram of anther size measurement. Scale bars, 1 mm. (e) The size of mature anther. Mean ± standard deviation values were calculated from ten biological repetition. There’s no significant difference between the samples at 26 °C and 15 °C (P > 0.05; Welch’s t test). (JPEG 346 kb)
Supplementary Fig. S2. Anther endodermis secondary cell wall thickness at 26
°C and 15 °C. (a-b) Transverse section of anther endodermis cells at 26 °C (a) and 15 °C (b). Red arrow indicates the thickened secondary cell wall (scw). Scale bars, 2 μm. (c) Secondary cell wall thickness. One hundred cells from ten anthers were measured at each temperature condition. Mean ± standard deviation values were calculated from 100 repetition. There’s no significant difference between the samples at 26 °C and 15 °C (P > 0.05; Welch’s t-test). (JPEG 381 kb)
Supplementary Fig. S3. PCD in watermelon anther at microspore stages by TUNEL assays.
Fluorescence microscope of cross-sections of watermelon anthers at 26 °C (a-f) and 15 °C (g-l). Green fluorescence indicates TUNEL-positive signals while red fluorescence indicates DAPI (4’,6-diamidino-2-phenylindole) staining. White arrows indicate tapetal cells tissues undergoing PCD. Except the autofluorescence from watermelon pollen wall, no green fluorescence signal was observable in the anther at young microspore stage at both 26 °C (a-c) and 15 °C (g-i). At later microspore stage, TUNELpositive signals were present in tapetum cells at 26 °C (d-f), suggesting that advanced PCD degeneration had occurred (white arrows), while no signal was present in anthers at 15 °C at later microspore stage (j-l). Scale bars, 50 μm. (JPEG 763 kb)
Supplementary Fig. S4. Pollen viability at 26
°C and 15 °C. (a-d) Viability of pollen grains as assayed with Alexander staining. Pollen grains collected from the mature anthers at both 26 °C (a) and 15 °C (c), as well as those collected from dehiscent anthers at 26 °C (b) were viable (purple-stained pollen grains), while that of the pollen grains collected from the indehiscent anthers after pollen mature stage at 15 °C (d) contains a lot of non-viable pollen grains (blue-stained pollen grains; red arrows). mp: mature pollen; pad: pollen after anther dehiscence; pia: pollen in the indehiscent anthers. Scale bars, 100 μm. (e) Reduced pollen viability in indehiscent anthers at 15 °C. Pollen grains were observed in three different fields of view with 10 biological repetitions. Results were plotted as mean ± standard deviation values calculated from 30 repetitions in percentage of pollen viability. The viability of pollen from indehiscent anther at 15 °C was significantly reduced compared to the pollen from dehiscent anthers at 26 °C. (***P < 0.001; Welch’s t-test). (JPEG 1174 kb)
Rights and permissions
About this article
Cite this article
Lyu, X., Chen, S., Liao, N. et al. Characterization of watermelon anther and its programmed cell death-associated events during dehiscence under cold stress. Plant Cell Rep 38, 1551–1561 (2019). https://doi.org/10.1007/s00299-019-02466-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-019-02466-2