Abstract
Key message
Our results based on proteomics data and physiological alterations proposed the putative mechanism of exogenous Spd enhanced salinity tolerance in cucumber seedlings.
Abstract
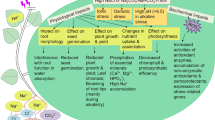
Current studies showed that exogenous spermidine (Spd) could alleviate harmful effects of salinity. It is important to increase our understanding of the beneficial physiological responses of exogenous Spd treatment, and to determine the molecular responses underlying these responses. Here, we combined a physiological analysis with iTRAQ-based comparative proteomics of cucumber (Cucumis sativus L.) leaves, treated with 0.1 mM exogenous Spd, 75 mM NaCl and/or exogenous Spd. A total of 221 differentially expressed proteins were found and involved in 30 metabolic pathways, such as photosynthesis, carbohydrate metabolism, amino acid metabolism, stress response, signal transduction and antioxidant. Based on functional classification of the differentially expressed proteins and the physiological responses, we found cucumber seedlings treated with Spd under salt stress had higher photosynthesis efficiency, upregulated tetrapyrrole synthesis, stronger ROS scavenging ability and more protein biosynthesis activity than NaCl treatment, suggesting that these pathways may promote salt tolerance under high salinity. This study provided insights into how exogenous Spd protects photosynthesis and enhances salt tolerance in cucumber seedlings.






Similar content being viewed by others
Abbreviations
- ALA:
-
5-Aminolevulinic acid
- Ci:
-
Intercellular CO2
- FW:
-
Fresh weight
- Gs:
-
Stomatal conductance
- iTRAQ:
-
Isobaric tags for relative and absolute quantification
- NPQ:
-
Non-photochemical quenching
- PAs:
-
Polyamines
- Pn:
-
Net photosynthetic rate
- ROS:
-
Reactive oxygen species
- Spd:
-
Spermidine
- TCA:
-
Tricarboxylic acid cycle
- OPP:
-
Oxidative pentose phosphate pathway
References
Abbasi FM, Komatsu S (2004) A proteomic approach to analyze salt-responsive proteins in rice leaf sheath. Proteomics 4:2072–2081
Akram NA, Ashraf M (2013) Regulation in plant stress tolerance by a potential plant growth regulator, 5-aminolevulinic acid. J Plant Growth Regul 32:663–679
Akram NA, Ashraf M, Al-Qurainy F (2012) Aminolevulinic acid-induced changes in some key physiological attributes and activities of antioxidant enzymes in sunflower (Helianthus annuus L.) plants under saline regimes. Sci Hortic-amsterdam 142:143–148
Alcázar R, Altabella T, Marco F, Bortolotti C, Reymond M, Koncz C, Carrasco P, Tiburcio AF (2010) Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231:1237–1249
Amarie S, Wilk L, Barros T, Kühlbrandt W, Dreuw A, Wachtveitl J (2009) Properties of zeaxanthin and its radical cation bound to the minor light-harvesting complexes CP24, CP26 and CP29. BBA-Bioenergetics 1787:747–752
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141:391–396
Azevedo C, Santos-Rosa MJ, Shirasu K (2001) The U-box protein family in plants. Trends Plant Sci 6:354–358
Bauwe H, Hagemann M, Fernie AR (2010) Photorespiration: players, partners and origin. Trends Plant Sci 15:330–336
Begley TP, Kinsland C, Strauss E (2001) The biosynthesis of coenzyme A in bacteria. Vitam Horm 61:157–171
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brian D, Ian B (1984) Estimation of hydrogen peroxide in plant extracts using titanium. Anal Chem 139:487–492
Brown NM, Torres AS, Doan PE, O’Halloran TV (2004) Oxygen and the copper chaperone CCS regulate posttranslational activation of Cu, Zn superoxide dismutase. Proc Natl Acad Sci 101:5518–5523
Chattopadhayay MK, Tiwari BS, Chattopadhyay G, Bose A, Sengupta DN, Ghosh B (2002) Protective role of exogenous polyamines on salinity-stressed rice (Oryza sativa) plants. Physiol Plant 116:192–199
Chaves M, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560
Chen D, Ma X, Li C, Zhang W, Xia G, Wang M (2014) A wheat aminocyclopropane-1-carboxylate oxidase gene, TaACO1, negatively regulates salinity stress in Arabidopsis thaliana. Plant Cell Rep 33:1815–1827
Cheng F, Zhou Y-H, Xia X-J, Shi K, Zhou J, Yu J-Q (2014) Chloroplastic thioredoxin-f and thioredoxin-m1/4 play important roles in brassinosteroids-induced changes in CO2 assimilation and cellular redox homeostasis in tomato. J Exp Bot 65:4335–4347
Chu C-C, Lee W-C, Guo W-Y, Pan S-M, Chen L-J, H-m Li, Jinn T-L (2005) A copper chaperone for superoxide dismutase that confers three types of copper/zinc superoxide dismutase activity in Arabidopsis. Plant Physiol 139:425–436
Duan J, Li J, Guo S, Kang Y (2008) Exogenous spermidine affects polyamine metabolism in salinity-stressed Cucumis sativus roots and enhances short-term salinity tolerance. J Plant Physiol 165:1620–1635
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal Biochem 70:616–620
Fan P, Feng J, Jiang P, Chen X, Bao H, Nie L, Jiang D, Lv S, Kuang T, Li Y (2011) Coordination of carbon fixation and nitrogen metabolism in Salicornia europaea under salinity: comparative proteomic analysis on chloroplast proteins. Proteomics 11:4346–4367
Geigenberger P, Hajirezaei M, Geiger M, Deiting U, Sonnewald U, Stitt M (1998) Overexpression of pyrophosphatase leads to increased sucrose degradation and starch synthesis, increased activities of enzymes for sucrose-starch interconversions, and increased levels of nucleotides in growing potato tubers. Planta 205:428–437
Geisler M, Bailly A (2007) Tete-a-tete: the function of FKBPs in plant development. Trends Plant Sci 12:465–473
George GM, Van Der Merwe MJ, Nunes-Nesi A, Bauer R, Fernie AR, Kossmann J, Lloyd JR (2010) Virus-induced gene silencing of plastidial soluble inorganic pyrophosphatase impairs essential leaf anabolic pathways and reduces drought stress tolerance in Nicotiana benthamiana. Plant Physiol 154:55–66
Groppa M, Benavides M (2008) Polyamines and abiotic stress: recent advances. Amino Acids 34:35–45
John RA (1995) Pyridoxal phosphate-dependent enzymes. BBA-Protein. Struct M 1248:81–96
Kalanon M, McFadden GI (2008) The chloroplast protein translocation complexes of Chlamydomonas reinhardtii: a bioinformatic comparison of Toc and Tic components in plants, green algae and red algae. Genetics 179:95–112
Kamal AHM, Cho K, Kim D-E, Uozumi N, Chung K-Y, Lee SY, Choi J-S, Cho S-W, Shin C-S, Woo SH (2012) Changes in physiology and protein abundance in salt-stressed wheat chloroplasts. Mol Biol Rep 39:9059–9074
Kasukabe Y, He L, Nada K, Misawa S, Ihara I, Tachibana S (2004) Overexpression of spermidine synthase enhances tolerance to multiple environmental stresses and up-regulates the expression of various stress-regulated genes in transgenic Arabidopsis thaliana. Plant Cell Physiol 45:712–722
Kumria R, Rajam MV (2002) Ornithine decarboxylase transgene in tobacco affects polyamines, in vitro-morphogenesis and response to salt stress. J Plant Physiol 159:983–990
Kurisu G, Zhang H, Smith JL, Cramer WA (2003) Structure of the cytochrome b6f complex of oxygenic photosynthesis: tuning the cavity. Science 302:1009–1014
Laule O, Fürholz A, Chang H-S, Zhu T, Wang X, Heifetz PB, Gruissem W, Lange M (2003) Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci 100:6866–6871
Liu G-T, Ma L, Duan W, Wang B-C, Li J-H, Xu H-G, Yan X-Q, Yan B-F, Li S-H, Wang L-J (2014) Differential proteomic analysis of grapevine leaves by iTRAQ reveals responses to heat stress and subsequent recovery. BMC Plant Biol 14:110
Lohse M, Nagel A, Herter T, May P, Schroda M, Zrenner R, Tohge T, Fernie AR, Stitt M, Usadel B (2014) Mercator: a fast and simple web server for genome scale functional annotation of plant sequence data. Plant, Cell Environ 37:1250–1258
Miller G, Suzuki N, CIFTCI-YILMAZ S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant, Cell Environ 33:453–467
Mittenhuber G (2001) Phylogenetic analyses and comparative genomics of vitamin B6 (pyridoxine) and pyridoxal phosphate biosynthesis pathways. J Mol Microbiol Biotechnol 3:1–20
Momcilovic I, Ristic Z (2007) Expression of chloroplast protein synthesis elongation factor, EF-Tu, in two lines of maize with contrasting tolerance to heat stress during early stages of plant development. J Plant Physiol 164:90–99
Moschou PN, Paschalidis KA, Delis ID, Andriopoulou AH, Lagiotis GD, Yakoumakis DI, Roubelakis-Angelakis KA (2008) Spermidine exodus and oxidation in the apoplast induced by abiotic stress is responsible for H2O2 signatures that direct tolerance responses in tobacco. Plnat Cell 20:1708–1724
Nagahatenna DS, Langridge P, Whitford R (2015) Tetrapyrrole-based drought stress signalling. Plant Biotechnol J 13:447–459
Porra R, Thompson W, Kriedemann P (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. BBA-Bioenergetics 975:384–394
Raines CA (2003) The Calvin cycle revisited. Photosynth Res 75:1–10
Robinson SP, Downton WJS, Millhouse JA (1983) Photosynthesis and ion content of leaves and isolated chloroplasts of salt-stressed spinach. Plant Physiol 73:238–242
Roychoudhury A, Basu S, Sengupta DN (2011) Amelioration of salinity stress by exogenously applied spermidine or spermine in three varieties of indica rice differing in their level of salt tolerance. J Plant Physiol 168:317–328
Rubio S, Whitehead L, Larson TR, Graham IA, Rodriguez PL (2008) The coenzyme a biosynthetic enzyme phosphopantetheine adenylyltransferase plays a crucial role in plant growth, salt/osmotic stress resistance, and seed lipid storage. Plant Physiol 148:546–556
Sharkia R, Bonshtien AL, Mizrahi I, Weiss C, Niv A, Lustig A, Viitanen PV, Azem A (2003) On the oligomeric state of chloroplast chaperonin 10 and chaperonin 20. BBA-Proteins Proteom 1651:76–84
Shi H, Ye T, Chan Z (2013) Comparative proteomic and physiological analyses reveal the protective effect of exogenous polyamines in the bermudagrass (Cynodon dactylon) response to salt and drought stresses. J Proteome Res 12:4951–4964
Shilov IV, Seymour SL, Patel AA, Loboda A, Tang WH, Keating SP, Hunter CL, Nuwaysir LM, Schaeffer DA (2007) The Paragon Algorithm, a next generation search engine that uses sequence temperature values and feature probabilities to identify peptides from tandem mass spectra. Mol Cell Proteomics 6:1638–1655
Shu S, Chen L, Lu W, Sun J, Guo S, Yuan Y, Li J (2014) Effects of exogenous spermidine on photosynthetic capacity and expression of Calvin cycle genes in salt-stressed cucumber seedlings. J Plant Res 127:763–773
Slocum RD, Kaur-Sawhney R, Galston AW (1984) The physiology and biochemistry of polyamines in plants. Arch Biochem Biophys 235:283–303
Stepien P, Johnson GN (2009) Contrasting responses of photosynthesis to salt stress in the glycophyte Arabidopsis and the halophyte Thellungiella: role of the plastid terminal oxidase as an alternative electron sink. Plant Physiol 149:1154–1165
Sudhir P, Murthy S (2004) Effects of salt stress on basic processes of photosynthesis. Photosynthetica 42:481–486
Suzuki H, Ueda T, Taguchi H, Takeuchi N (2007) Chaperone properties of mammalian mitochondrial translation elongation factor Tu. J Biol Chem 282:4076–4084
Takahashi S, Badger MR (2011) Photoprotection in plants: a new light on photosystem II damage. Trends Plant Sci 16:53–60
Takahashi S, Murata N (2008) How do environmental stresses accelerate photoinhibition? Trends Plant Sci 13:178–182
Tanou G, Ziogas V, Belghazi M, Christou A, Filippou P, Job D, Fotopoulos V, Molassiotis A (2014) Polyamines reprogram oxidative and nitrosative status and the proteome of citrus plants exposed to salinity stress. Plant, Cell Environ 37:864–885
Titiz O, Tambasco-Studart M, Warzych E, Apel K, Amrhein N, Laloi C, Fitzpatrick TB (2006) PDX1 is essential for vitamin B6 biosynthesis, development and stress tolerance in Arabidopsis. Plant J 48:933–946
Triantaphylidès C, Havaux M (2009) Singlet oxygen in plants: production, detoxification and signaling. Trends Plant Sci 14:219–228
Tsakraklides G, Martin M, Chalam R, Tarczynski MC, Schmidt A, Leustek T (2002) Sulfate reduction is increased in transgenic Arabidopsis thaliana expressing 5′-adenylylsulfate reductase from Pseudomonas aeruginosa. Plant J 32:879–889
Tuteja N (2007) Chapter twenty-four-mechanisms of high salinity tolerance in plants. Methods Enzymol 428:419–438
Vandenabeele S, Vanderauwera S, Vuylsteke M, Rombauts S, Langebartels C, Seidlitz HK, Zabeau M, Van Montagu M, Inzé D, Van Breusegem F (2004) Catalase deficiency drastically affects gene expression induced by high light in Arabidopsis thaliana. Plant J 39:45–58
Wang L, Liang W, Xing J, Tan F, Chen Y, Huang L, Cheng C-L, Chen W (2013) Dynamics of chloroplast proteome in salt-stressed mangrove Kandelia candel (L.) Druce. J Proteome Res 12:5124–5136
Wi SJ, Kim WT, Park KY (2006) Overexpression of carnation S-adenosylmethionine decarboxylase gene generates a broad-spectrum tolerance to abiotic stresses in transgenic tobacco plants. Plant Cell Rep 25:1111–1121
Yoo KS, Ok SH, Jeong B-C, Jung KW, Cui MH, Hyoung S, Lee M-R, Song HK, Shin JS (2011) Single cystathionine β-synthase domain–containing proteins modulate development by regulating the thioredoxin system in Arabidopsis. Plnat Cell 23:3577–3594
Youssefian S, Nakamura M, Orudgev E, Kondo N (2001) Increased cysteine biosynthesis capacity of transgenic tobacco overexpressing an O-acetylserine (thiol) lyase modifies plant responses to oxidative stress. Plant Physiol 126:1001–1011
Zhang RH, Li J, Guo SR, Tezuka T (2009) Effects of exogenous putrescine on gas-exchange characteristics and chlorophyll fluorescence of NaCl-stressed cucumber seedlings. Photosynth Res 100:155–162
Zhang H, Han B, Wang T, Chen S, Li H, Zhang Y, Dai S (2011) Mechanisms of plant salt response: insights from proteomics. J Proteome Res 11:49–67
Zhang P, Li C, Zhang P, Jin C, Pan D, Bao Y (2014) iTRAQ-based proteomics reveals novel members involved in pathogen challenge in sea cucumber Apostichopus japonicus. PLoS One 9:e100492
Zhang Z-W, Zhang G-C, Zhu F, Zhang D-W, Yuan S (2015) The roles of tetrapyrroles in plastid retrograde signaling and tolerance to environmental stresses. Planta 242:1263–1276
Zhu J-K (2001) Plant salt tolerance. Trends Plant Sci 6:66–71
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos. 31401919, 31471869 and 31272209), the Central Research Institutes of Basic Research Fund (6J0745), the China Earmarked Fund for Modern Agro-industry Technology Research System (CARS-25-C-03), the Jiangsu Province Scientific and Technological Achievements into Special Fund (BA2014147), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by Qiao Zhao.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Effects of exogenous spermidine application on superoxide production rate and hydrogen peroxide content in leaves of cucumber under salt stress for 3 d. The Fig is representative of two independent experiments. Values represent mean ± SD (n = 6). Different letters indicate that they are significantly different from each other (P < 0.05). Determination method as previously described (Brian and Ian 1984; Elstner and Heupel 1976) (JPEG 923 kb)
Table S1
Differentially expressed cucumber proteins under exogenous Spd with/without NaCl treatment. (XLS 70 kb)
Table S2
Details of peptides in proteins whose response to NaCl or exogenous Spd with/without NaCl treatment. (XLS 4782 kb)
Rights and permissions
About this article
Cite this article
Sang, T., Shan, X., Li, B. et al. Comparative proteomic analysis reveals the positive effect of exogenous spermidine on photosynthesis and salinity tolerance in cucumber seedlings. Plant Cell Rep 35, 1769–1782 (2016). https://doi.org/10.1007/s00299-016-1995-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-016-1995-x