Abstract
Key message
Overexpression of Sp-miR396a-5p in tobacco increased tolerance to salt, drought, cold stress and susceptibility to Phytophthora nicotianae infection.
Abstract
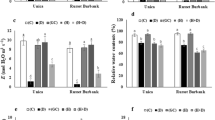
MicroRNA396 (miR396) is one of the conserved microRNA families in plants, and it targeted growth-regulating factors (GRFs) family. The GRF transcription factors are associated with growth and stress responses. However, the molecular mechanisms of miR396 responding to environmental stresses are elusive. The purpose of this study was to explore the function of tomato miR396a-5p (Sp-miR396a-5p) in Solanaceae responses to abiotic and biotic stresses. We showed that Sp-miR396a-5p transcript levels were up-regulated under salt and drought stresses and down-regulated after Phytophthora infestans (P. infestans) infection. Consistently, overexpression of Sp-miR396a-5p in tobacco enhanced its tolerance to salt, drought and cold stresses. Additionally, the expression of Sp-miR396a-5p was found to be down-regulated under pathogen-related biotic stress. Tobacco plants overexpressing Sp-miR396a-5p showed increased susceptibility to Phytophthora nicotianae (P. nicotianae) infection. Physiological analysis indicated that Sp-miR396a-5p overexpression enhanced osmoregulation and decreased production of reactive oxygen species (ROS). Furthermore, four Sp-miR396a-5p target genes, NtGRF1, NtGRF3, NtGRF7 and NtGRF8, were down-regulated in these plants. Our results suggested that Sp-miR396a-5p plays critical roles in both abiotic stresses through targeting NtGRF7-regulated expression of osmotic stress-responsive genes and pathogen infection via the regulatory networks of NtGRF1 and NtGRF3.








Similar content being viewed by others
References
Bazin J, Khan GA, Combier J-P, Bustos-Sanmamed P, Debernardi JM, Rodriguez R, Sorin C, Palatnik J, Hartmann C, Crespi M, Lelandais-Brière C (2013) miR396 affects mycorrhization and root meristem activity in the legume Medicago truncatula. Plant J 74:920–934
Cao X, Wu Z, Jiang F, Zhou R, Yang Z (2014) Identification of chilling stress-responsive tomato microRNAs and their target genes by high-throughput sequencing and degradome analysis. BMC Genom 15:1130
Casadevall R, Rodriguez RE, Debernardi JM, Palatnik JF, Casati P (2013) Repression of growth regulating factors by the MicroRNA396 inhibits cell proliferation by UV-B radiation in Arabidopsis leaves. Plant Cell 25:3570–3583
Chiou TJ, Aung K, Lin SI, Wu CC, Chiang SF, Su CL (2006) Regulation of phosphate homeostasis by microRNA in Arabidopsis. Plant Cell 18:412–421
Choi J, Choi D, Lee S, Ryu CM, Hwang I (2011) Cytokinins and plant immunity: old foes or new friends? Trends Plant Sci 16:388–394
Corbisier P, Houbion A, Remacle J (1987) A new technique for highly sensitive detection of superoxide dismutase activity by chemiluminescence. Analytical Biochem 164:240–247
Cui J, Luan Y, Wang W, Zhai J (2014) Prediction and validation of potential pathogenic microRNAs involved in Phytophthora infestans infection. Mol Biol Rep 41:1879–1889
Dai X, Zhao P (2011) psRNATarget: a plant small RNA target analysis server. Nucleic Acids Res 39:W155–W159
Fahlgren N, Howell MD, Kasschau KD, Chapman EJ, Sullivan CM, Cumbie JS, Givan SA, Law TF, Grant SR, Dangl JL, Carrington JC (2007) High-throughput sequencing of Arabidopsis microRNAs: evidence for frequent birth and death of MIRNA genes. PLoS One 2:e219
Hebbelmann I, Selinski J, Wehmeyer C, Goss T, Voss I, Mulo P, Kangasjärvi S, Aro EM, Oelze ML, Dietz KJ, Nunes-Nesi Adriano, Do PT, Fernie AR, Talla SK, Raghavendra AS, Linke V, Scheibe R (2012) Multiple strategies to prevent oxidative stress in Arabidopsis plants lacking the malate valve enzyme NADP-malate dehydrogenase. J Exp Bot 63:1445–1459
Hewezi T, Howe P, Maier TR, Baum TJ (2008) Arabidopsis small RNAs and their targets during cyst nematode parasitism. Mol Plant Microbe In 21:1622–1634
Hewezi T, Maier TR, Nettleton D, Baum TJ (2012) The Arabidopsis microRNA396-GRF1/GRF3 regulatory module acts as a developmental regulator in the reprogramming of root cells during cyst nematode infection. Plant Physiol 159:321–335
Kesarwani M, Yoo JM, Dong XN (2007) Genetic interactions of TGA transcription factors in the regulation of pathogenesis-related genes and disease resistance in Arabidopsis. Plant Physiol 144:336–346
Khraiwesh B, Zhu JK, Zhu J (2012) Role of miRNAs and siRNAs in biotic and abiotic stress responses of plants. Biochim Biophys Acta 1819:137–148
Kim JS, Mizoi J, Kidokoro S, Maruyama K, Nakajima J, Nakashima K, Mitsuda N, Takiguchi Y, Ohme-Takagi M, Kondou Y, Yoshizumi T, Matsui M, Shinozaki K, Yamaguchi-Shinozaki K (2012) Arabidopsis growth-regulating factor7 functions as a transcriptional repressor of abscisic acid- and osmotic stress-responsive genes, including DREB2A. Plant Cell 24:3393–3405
Kozomara A, Griffiths-Jones S (2014) miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res 42:D68–D73
Kravchik M, Sunkar R, Damodharan S, Stav R, Zohar M, Isaacson T, Arazi T (2014) Global and local perturbation of the tomato microRNA pathway by a trans-activated DICER-LIKE 1 mutant. J Exp Bot 65:725–739
Lee B, Lee H, Xiong L, Zhu JK (2002) A mitochondrial complex I defect impairs cold-regulated nuclear gene expression. Plant Cell 14:1235–1251
Li Y, Zhang Q, Zhang J, Wu L, Qi Y, Zhou J (2010a) a) Identification of microRNAs involved in pathogen-associated molecular pattern-triggered plant innate immunity. Plant Physiol 152:2222–2231
Li Z, Baldwin CM, Hu Q, Liu H, Luo H (2010b) b) Heterologous expression of Arabidopsis H + -pyrophosphatase enhances salt tolerance in transgenic creeping bentgrass (Agrostis stolonifera L.). Plant Cell Environ 33:272–289
Li J, Luan Y, Liu Z (2015) Overexpression of SpWRKY1 promotes resistance to Phytophthora nicotianae and tolerance to salt and drought stress in transgenic tobacco. Physiol Plantrum. doi:10.1111/ppl.12315
Liu H, Tian X, Li Y, Wu C, Zheng C (2008) Microarray-based analysis of stress-regulated microRNAs in Arabidopsis thaliana. RNA 14:836–843
Liu D, Song Y, Chen Z, Yu D (2009) Ectopic expression of miR396 suppresses GRF target gene expression and alters leaf growth in Arabidopsis. Physiol Plantrum 136:223–236
Liu X, Williams CE, Nemacheck JA, Wang H, Subramanyam S, Zheng C, Chen MS (2010) Reactive oxygen species are involved in plant defense against a gall midge. Plant Physiol 152:985–999
Liu J, Rice JH, Chen N, Baum TJ, Hewezi T (2014) Synchronization of developmental processes and defense signaling by growth regulating transcription factors. PLoS One 9:e98477
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Lu W, Chu X, Li Y, Wang C, Guo X (2013) Cotton GhMKK1 induces the tolerance of salt and drought stress, and mediates defence responses to pathogen infection in transgenic Nicotiana benthamiana. PLoS One 8:e68503
Luan Y, Wang W, Liu P (2014) Identification and functional analysis of novel and conserved microRNAs in tomato. Mol Biol Rep 41:5385–5394
Luan Y, Cui J, Zhai J, Li J, Han L, Meng J (2015) High-throughput sequencing reveals differential expression of miRNAs in tomato inoculated with Phytophthora infestans. Planta 241:1405–1416
Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasiri N, Estelle M, Voinnet O, Jones JD (2006) A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 21:436–439
Polle A, Otter T, Seifert F (1994) Apoplastic peroxidases and lignification in needles of Norway spruce (Picea abies L.). Plant Physiol 106:53–60
Rusk N (2015) Understanding noncoding RNAs. Nat Methods. doi:10.1038/nmeth.3235
Shao HB, Chen XY, Chu LY, Zhao XN, Wu G, Yuan YB, Zhao CX, Hu ZM (2006) Investigation on the relationship of proline with wheat anti-drought under soil water deficits. Colloid Surface B 53:113–119
Shearer HL, Cheng YT, Wang L, Liu J, Boyle P, Desprs C, Zhang Y, Li X, Fobert PR (2012) Arabidopsis clade I TGA transcription factors regulate plant defenses in an NPR1-independent fashion. Mol Plant Microbe In 25:1459–1468
Shen J, Xing T, Yuan H, Liu Z, Jin Z, Zhang L, Pei Y (2013) Hydrogen sulfide improves drought tolerance in Arabidopsis thaliana by MicroRNA expressions. PLoS One 8:e77047
Sun Y, Wang B, Jin S, Qu X, Li Y, Hou B (2013) Ectopic expression of Arabidopsis glycosyltransferase UGT85A5 enhances salt stress tolerance in tobacco. PLoS One 8:e59924
Sunkar R, Zhu JK (2004) Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 16:2001–2019
Sunkar R, Chinnusamy V, Zhu J, Zhu JK (2007) Small RNAs as big players in plant abiotic stress responses and nutrient deprivation. Trends Plant Sci 12:301–309
Sunkar R, Li YF, Jagadeeswaran G (2012) Functions of microRNAs in plant stress responses. Trends Plant Sci 17:196–203
Wang W, Luan Y (2015) The advance of tomato disease-related microRNAs. Plant Cell Rep 34:1089–1097
Wang L, Gu X, Xu D, Wang W, Wang H, Zeng M, Chang Z, Huang H, Cui X (2011) miR396-targeted AtGRF transcription factors are required for coordination of cell division and differentiation during leaf development in Arabidopsis. J Exp Bot 62:761–773
Wang M, Wang Q, Zhang B (2013) Response of miRNAs and their targets to salt and drought stresses in cotton (Gossypium hirsutum L.). Gene 530:26–32
Xin M, Wang Y, Yao Y, Xie C, Peng H, Ni Z, Sun Q (2010) Diverse set of microRNAs are responsive to powdery mildew infection and heat stress in wheat (Triticum aestivum L.). BMC Plant Biol 10:123
Yamada M, Morishita H, Urano K, Shiozaki N, Yamaguchi Shinozaki K, Shinozaki K, Yoshiba Y (2005) Effects of free proline accumulation in petunias under drought stress. J Exp Bot 56:1975–1981
Yang F, Yu D (2009) Overexpression of Arabidopsis miR396 enhances drought tolerance in transgenic tobacco plants. Acta Botanica Yunnanica 31:421–426
Yang F, Liang G, Liu D, Yu D (2009) Arabidopsis miR396 mediates the development of Leaves and flowers in transgenic tobacco. J Plant Biol 52:475–481
Zhang L, Li Y, Lu W, Meng F, Wu C, Guo X (2012) Cotton GhMKK5 affects disease resistance, induces HR-like cell death, and reduces the tolerance to salt and drought stress in transgenic Nicotiana benthamiana. J Exp Bot 63:3935–3951
Zhou X, Wang G, Sutoh K, Zhu J, Zhang W (2008) Identification of cold-inducible MicroRNAs in plants by transcriptome analysis. Biochim Biophys Acta 1779:780–788
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (31272167, 31471880 and 61472061).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest in the present investigation.
Additional information
Communicated by L. Peña.
Rights and permissions
About this article
Cite this article
Chen, L., Luan, Y. & Zhai, J. Sp-miR396a-5p acts as a stress-responsive genes regulator by conferring tolerance to abiotic stresses and susceptibility to Phytophthora nicotianae infection in transgenic tobacco. Plant Cell Rep 34, 2013–2025 (2015). https://doi.org/10.1007/s00299-015-1847-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-015-1847-0