Abstract
Key message
Virus-induced gene silencing (VIGS) system could be performed successfully in Gladiolus hybridus with vacuum infiltration of cormels and young plants.
Abstract
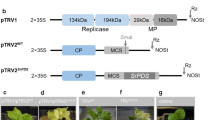
Functional analysis of genes in gladiolus has previously been impractical due to the lack of an efficient stable genetic transformation method. However, virus-induced gene silencing (VIGS) is effective in some plants which are difficult to transform through other methods. Although the Tobacco rattle virus (TRV)-based VIGS system has been developed and used for verifying gene functions in diverse plants, an appropriate TRV-VIGS approach for gladiolus has not been established yet. In this report we describe the first use of the TRV-VIGS system for gene silencing in gladiolus. Vacuum infiltration of cormels and young plants with the GhPDS-VIGS vector effectively down-regulated the PHYTOENE DESATURASE ortholog GhPDS gene and also resulted in various degrees of photobleaching in Gladiolus hybridus. The reduction in GhPDS expression was tested after TRV-based vector infection using real-time RT-PCR. In addition, the progress of TRV infection was detected by fluorescence visualization using a pTRV2: CP-GFP vector. In conclusion, the TRV-mediated VIGS described here will be an effective gene function analysis mechanism in gladiolus.








Similar content being viewed by others
References
An G (1985) High efficiency transformation of cultured tobacco cells. Plant Physiol 79(2):568–570
An G, Watson BD, Chiang CC (1986) Transformation of tobacco, tomato, potato, and Arabidopsis thaliana using a binary Ti vector system. Plant Physiol 81(1):301–305
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297
Baulcombe DC (1999) Fast forward genetics based on virus-induced gene silencing. Curr Opin Plant Biol 2(2):109–113
Baulcombe DC, Chapman S, Cruz S (1995) Jellyfish green fluorescent protein as a reporter for virus infections. Plant J 7(6):1045–1053
Bruun-Rasmussen M, Madsen CT, Jessing S, Albrechtsen M (2007) Stability of barley stripe mosaic virus-induced gene silencing in barley. Mol Plant Microbe Interact 20(11):1323–1331
Burch-Smith TM, Anderson JC, Martin GB, Dinesh-Kumar SP (2004) Applications and advantages of virus-induced gene silencing for gene function studies in plants. Plant J 39(5):734–746
Chen YY, Lin YM, Chao TC, Wang JF, Liu AC, Ho FI, Cheng CP (2009) Virus-induced gene silencing reveals the involvement of ethylene-, salicylic acid- and mitogen-activated protein kinase-related defense pathways in the resistance of tomato to bacterial wilt. Physiol Plant 136(3):324–335
Conner A, Dommisse E (1992) Monocotyledonous plants as hosts for Agrobacterium. Int J Plant Sci 153(4):550–555
Cunningham F Jr, Gantt E (1998) Genes and enzymes of carotenoid biosynthesis in plants. Annu Rev Plant Biol 49(1):557–583
Demircan T, Akkaya MS (2010) Virus induced gene silencing in Brachypodium distachyon, a model organism for cereals. Plant Cell, Tissue Organ Cult 100(1):91–96
Desfeux C, Clough SJ, Bent AF (2000) Female reproductive tissues are the primary target of Agrobacterium-mediated transformation by the Arabidopsis floral-dip method. Plant Physiol 123(3):895–904
Di Stilio VS, Kumar RA, Oddone AM, Tolkin TR, Salles P, McCarty K (2010) Virus-induced gene silencing as a tool for comparative functional studies in Thalictrum. PLoS ONE 5(8):e12064
Dinesh-Kumar S, Anandalakshmi R, Marathe R, Schiff M, Liu Y (2003) Virus-induced gene silencing. Methods Mol Biol 236:287–294
Faivre-Rampant O, Gilroy EM, Hrubikova K, Hein I, Millam S, Loake GJ, Birch P, Taylor M, Lacomme C (2004) Potato virus X-induced gene silencing in leaves and tubers of potato. Plant Physiol 134(4):1308–1316
Gould B, Kramer EM (2007) Virus-induced gene silencing as a tool for functional analyses in the emerging model plant Aquilegia (columbine, Ranunculaceae). Plant Methods 3(1):6
Grønlund M, Olsen A, Johansen EI, Jakobsen I (2010) Protocol: using virus-induced gene silencing to study the arbuscular mycorrhizal symbiosis in Pisum sativum. Plant Methods 6(1):28
Hidalgo O, Bartholmes C, Gleissberg S (2012) Virus-induced gene silencing (VIGS) in Cysticapnos vesicaria, a zygomorphic-flowered Papaveraceae (Ranunculales, basal eudicots). Ann Bot 109(5):911–920
Hileman LC, Drea S, Martino G, Litt A, Irish VF (2005) Virus-induced gene silencing is an effective tool for assaying gene function in the basal eudicot species Papaver somniferum (opium poppy). Plant J 44(2):334–341
Holzberg S, Brosio P, Gross C, Pogue GP (2002) Barley stripe mosaic virus-induced gene silencing in a monocot plant. Plant J 30(3):315–327
Jiang CZ, Chen JC, Reid M (2011) RNAi and Plant Gene Function Analysis. In: Kodama H, Komamine A (eds) Virus-induced gene silencing in ornamental plants., Methods in Molecular BiologyHumana Press, New York, pp 81–96
Jones ML, Barnard RT (2005) Chimerization of multiple antibody classes using splice overlap extension PCR. Biotechniques 38:181–182
Jones L, Hamilton AJ, Voinnet O, Thomas CL, Maule AJ, Baulcombe DC (1999) RNA-DNA interactions and DNA methylation in post-transcriptional gene silencing. The Plant Cell Online 11(12):2291–2302
Kamo K, Jordan R, Guaragna MA, Hsu H-t, Ueng P (2010) Resistance to Cucumber mosaic virus in gladiolus plants transformed with either a defective replicase or coat protein subgroup II gene from Cucumber mosaic virus. Plant Cell Rep 29(7):695–704
Lacomme C, Hrubikova K (2003) Enhancement of virus-induced gene silencing through viral-based production of inverted-repeats. Plant J 34(4):543–553
Lian QL, Xin HB, Zhong XH, Zhang ZY, Li XX, Yuan X, Han HJ, He XL, Yi MF (2011) Cloning, characterization and expression analysis of a 9-lipoxygenase gene in Gladiolus hybridus. Sci Hortic 130(2):468–475
Liu YL, Schiff M, Dinesh-Kumar S (2002a) Virus-induced gene silencing in tomato. Plant J 31(6):777–786
Liu YL, Schiff M, Marathe R, Dinesh-Kumar S (2002b) Tobacco Rar1, EDS1 and NPR1/NIM1 like genes are required for N-mediated resistance to tobacco mosaic virus. Plant J 30(4):415–429
Liu HP, Fu DQ, Zhu BZ, Yan HX, Shen XY, Zuo JH, Zhu Y, Luo YB (2012) Virus-induced gene silencing in eggplant (Solanum melongena). J Integr Plant Biol 54(6):422–429
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408
Luo X, Yi J, Zhong XH, Lian QL, Khan MA, Cao X, Li XX, Gao MW, Wu J, Chen J (2012) Cloning, characterization and expression analysis of key genes involved in ABA metabolism in gladiolus cormels during storage. Sci Hortic 143:115–121
MacFarlane SA, Popovich AH (2000) Efficient expression of foreign proteins in roots from tobravirus vectors. Virology 267(1):29–35
Padmanabhan M, Dinesh-Kumar SP (2009) Virus-induced gene silencing as a tool for delivery of dsRNA into plants. Cold Spring Harbor Protocols 2009 (2):pdb. prot5139
Quadrana L, Rodriguez MC, López M, Bermúdez L, Nunes-Nesi A, Fernie AR, Descalzo A, Asis R, Rossi M, Asurmendi S (2011) Coupling virus-induced gene silencing to exogenous green fluorescence protein expression provides a highly efficient system for functional genomics in Arabidopsis and across all stages of tomato fruit development. Plant Physiol 156(3):1278–1291
Ratcliff F, Martin-Hernandez AM, Baulcombe DC (2001) Technical advance: tobacco rattle virus as a vector for analysis of gene function by silencing. Plant J 25(2):237–245
Scofield SR, Nelson RS (2009) Resources for virus-induced gene silencing in the grasses. Plant Physiol 149(1):152–157
Senthil-Kumar M, Mysore KS (2011) New dimensions for VIGS in plant functional genomics. Trends Plant Sci 16(12):656–665
Smith RH, Hood EE (1995) Agrobacterium tumefaciens transformation of monocotyledons. Crop Sci 35(2):301–309
Velásquez AC, Chakravarthy S, Martin GB (2009) Virus-induced gene silencing (VIGS) in Nicotiana benthamiana and tomato. J Vis Exp 28:1292
Visser R, Jacobsen E, Witholt B, Feenstra W (1989) Efficient transformation of potato (Solanum tuberosum L.) using a binary vector in Agrobacterium rhizogenes. Theor Appl Genet 78(4):594–600
Waterhouse PM, Wang M-B, Lough T (2001) Gene silencing as an adaptive defence against viruses. Nature 411(6839):834–842
Wege S, Scholz A, Gleissberg S, Becker A (2007) Highly efficient virus-induced gene silencing (VIGS) in California poppy (Eschscholzia californica): an evaluation of VIGS as a strategy to obtain functional data from non-model plants. Ann Bot 100(3):641–649
Yaegashi H, Yamatsuta T, Takahashi T, Li C, Isogai M, Kobori T, Ohki S, Yoshikawa N (2007) Characterization of virus-induced gene silencing in tobacco plants infected with apple latent spherical virus. Arch Virol 152(10):1839–1849
Yamagishi N, Yoshikawa N (2009) Virus-induced gene silencing in soybean seeds and the emergence stage of soybean plants with Apple latent spherical virus vectors. Plant Mol Biol 71(1):15–24
Yamagishi N, Yoshikawa N (2011) Virus-induced gene silencing of endogenous genes and promotion of flowering in soybean by Apple latent spherical virus-based vectors. Soybean-molecular aspects of breeding. InTech, Rijeka
Yang L, Wang H, Liu J, Li L, Fan Y, Wang X, Song Y, Sun S, Wang L, Zhu X (2008) A simple and effective system for foreign gene expression in plants via root absorption of agrobacterial suspension. J Biotechnol 134(3):320–324
Acknowledgments
We are grateful to Dr. Michael Reid and Dr. Cai-Zhong Jiang (University of California, Davis) for kindly offering pTRV1 and pTRV2 vectors. Our thanks also go to Charles Copeland (University of British Columbia) for helpful editing of this manuscript and to Lin Xi PhD (China Agricultural University) for helpful comments. This study has been supported by the Science and Technology Specific Project Foundation of Ministry of Agriculture, PR China (No. 200903020), National Natural Science Foundation of China (No. 31171991).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Kamo.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure S1 PDS sequence similarity analysis. (a) Sequence comparison of the coding region of PDS amplified from Gladiolus hybridus. Gladiolus hybridus (GhPDS, KC344859), Nicotiana benthamiana (NbPDS, DQ469932), Crocus sativus (CsPDS, AY183118), Oryza sativa (OsPDS, AF049356), Narcissus tazetta var. chinensis cultivar yellow (NtPDS, JQ797377) and Lilium hybrid division I (LiPDS, AB445118) (b) Phylogenetic tree.
Rights and permissions
About this article
Cite this article
Zhong, X., Yuan, X., Wu, Z. et al. Virus-induced gene silencing for comparative functional studies in Gladiolus hybridus . Plant Cell Rep 33, 301–312 (2014). https://doi.org/10.1007/s00299-013-1530-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-013-1530-2