Abstract
Key message
Two β-1,3-glucanase genes from sugarcane were cloned and characterized. They were all located in apoplast and involves in different expression patterns in biotic and abiotic stress.
Abstract
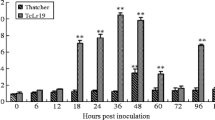
Smut caused by Sporisorium scitamineum is a serious disease in the sugarcane industry. β-1,3-Glucanase, a typical pathogenesis-related protein, has been shown to express during plant–pathogen interaction and involves in sugarcane defense response. In this study, β-1,3-glucanase enzyme activity in the resistant variety increased faster and lasted longer than that of the susceptible one when inoculated with S. scitamineum, along with a positive correlation between the activity of the β-1,3-glucanase and smut resistance. Furthermore, two β-1,3-glucanase genes from S. scitamineum infected sugarcane, ScGluA1 (GenBank Accession No. KC848050) and ScGluD1 (GenBank Accession No. KC848051) were cloned and characterized. Phylogenetic analysis suggested that ScGluA1 and ScGluD1 clustered within subfamily A and subfamily D, respectively. Subcellular localization analysis demonstrated that both gene products were targeted to apoplast. Escherichia coli Rosetta (DE3) cells expressing ScGluA1 and ScGluD1 showed varying degrees of tolerance to NaCl, CdCl2, PEG, CuCl2 and ZnSO4. Q-PCR analysis showed up-regulation of ScGluA1 and slight down-regulation of ScGluD1 in response to S. scitamineum infection. It suggested that ScGluA1 may be involved in the defense reaction of the sugarcane to the smut, while it is likely that ScGluD1 was inhibited. The gene expression patterns of ScGluA1 and ScGluD1, in response to abiotic stresses, were similar to sugarcane response against smut infection. Together, β-1,3-glucanase may function in sugarcane defense mechanism for S. scitamineum. The positive responses of ScGluA1 and the negative responses of ScGluD1 to biotic and abiotic stresses indicate they play different roles in interaction between sugarcane and biotic or abiotic stresses.












Similar content being viewed by others
References
Akiyama T, Pillai MA (2001) Molecular cloning, characterization and in vitro expression of a novel endo-1,3-β-glucanase up-regulated by ABA and drought stress in rice (Oryza sativa L.). Plant Sci 161:1089–1098
Alexander KC, Ramakrishnan K (1980) Infection of the bud, establishment in the host and production of whips in sugarcane smut (Ustilago scitaminea) of sugarcane. Proc Int Soc Sugar Cane Technol 17:1452–1455
Anderberg RA, Walker-Simmons MK (1992) Isolation of a wheat cDNA clone for an abscisic acid-inducible transcript with homology to protein kinases. Proc Natl Acad Sci 89:10183–10187
Boller T (1985) Induction of hydrolases as defense reaction against pathogens. In: Key JL, Kosuge T (eds) Mol cell biology. AR Liss, New York, pp 247–262
Chao CP, Hoy JW, Saxton AM, Martin FA (1990) Heritability of resistance and repeatability of clone reactions to sugarcane smut in Louisiana. Phytopathology 80:622–626
Chaurasia N, Mishra Y, Rai LC (2008) Cloning expression and analysis of phytochelatin synthase (pcs) gene from Anabaena sp. PCC 7120 offering multiple stress functional tolerance in Escherichia coli. Biochem Biophys Res Co 376:225–230
Cheong YH, Kim CY, Chun HJ, Moon BC, Park HC, Kim JK, Lee S, Han C, Lee SY, Cho MJ (2000) Molecular cloning of a soybean class III β-1,3-glucanase gene that is regulated both developmentally and in response to pathogen infection. Plant Sci 154:71–81
Damaj MB, Kumpatla SP, Emani C, Beremand PD, Reddy AS, Rathore KS, Buenrostro-Nava MT, Curtis IS, Thomas TL, Mirkov TE (2010) Sugarcane DIRIGENT and O-methyltransferase promoters confer stem-regulated gene expression in diverse monocots. Planta 231:1439–1458
Dussle CM, Quint M, Melchinger AE, Xu ML, Lubberstedt T (2003) Saturation of two chromosome regions conferring resistance to SCMV with SSR and AFLP markers by targeted BSA. Theor Appl Genet 106:485–493
Eugenia-Rivera M, Codina JC, Olea F, De-Vicente A, Perez-Garcia A (2002) Differential expression of β-1,3-glucanase in susceptible and resistant melon cultivars in response to infection by Sphaerotheca fusca. Physiol Mol Plant P 61:257–265
Gu LH, Zhang SZ, Yang BP, Cai WW, Huang DJ, Wang WZ, Li J (2008) Introduction of chitin and β-1,3-glucan into sugarcane. Chin Mol Plant Breed 6:277–280
Guo JL, Xu LP, Fang JP, Su YC, Fu HY, Que YX, Xu JS (2012) A novel dirigent protein gene with highly stem-specific expression from sugarcane, response to drought, salt and oxidative stresses. Plant Cell Rep 31:1801–1812
Gupta K, Agarwal PK, Reddy MK, Jha B (2010) SbDREB2A, an A-2 type DREB transcription factor from extreme halophyte Salicornia brachiata confers abiotic stress tolerance in Escherichia coli. Plant Cell Rep 29:1131–1137
Higa-Nishiyama A, Ohsato S, Banno S, Woo SH, Fujimura M, Yamaguchi I, Kimura M (2006) Cloning and characterization of six highly similar endo-1,3-β-glucanase genes in hexaploid wheat. Plant Physiol Biochem 44:666–673
Hoy JW, Hollier CA, Fontenot DB, Grelen LB (1986) Incidence of sugarcane smut in Louisiana and its effects on yield. Plant Dis 70:59–60
Jayaraj J, Muthukrishnan S, Liang GH, Velazhahan R (2004) Jasmonic acid and salicylic acid induce accumulation of β-1,3-glucanase and thaumatin-like proteins in wheat and enhance resistance against Stagonospora nodorum. Bio Plantarum 48:425–430
Ji C, Norton RA, Wicklow DT, Dowd PF (2000) Isoform patterns of chitinase and β-1,3-glucanase in maturing corn kernels (Zea mays L.) associated with Aspergillus flavus milk stage infection. J Agric Food Chem 48:507–511
Jondle DJ, Coors JG, Duke SH (1989) Maize leaf β-1,3-glucanase activity in relation to resistance to Exserohilum turcicum. Can J Bot 67:263–266
Kemp G, Botha AM, Kloppers FJ, Pretorius ZA (1999) Disease development and β-1,3-glucanase expression following leaf rust infection in resistant and susceptible near-isogenic wheat seedlings. Physiol Mol Plant P 55:45–52
Lakshmanan P, Geijskes RJ, Aitken KS, Grof CLP, Bonnett GD, Smith GR (2005) Sugarcane biotechnology: the challenges and opportunities. In Vitro Cell Dev Pl 41:345–363
Leubner-Metzger G, Meins F Jr (1999) Functions and regulation of plant β-1,3-glucanases (PR-2). In: Datta SK, Muthukrishnan S (eds) Pathogenesis-related proteins plants. CRC Press, Boca Raton, pp 49–76
Li DM, Staehelin C, Wang WT, Peng SL (2010a) Molecular cloning and characterization of a chitinase-homologous gene from Mikania micrantha infected by Cuscuta campestris. Plant Mol Biol Rep 28:90–101
Li SG, Gu JG, Jiang RB, Niu YC (2010b) β-1,3-glucanase activity from biocontrol Trichoderma strains. Chin J Microbiol 30:88–91
Lin YQ, Chen RK, Gong DM (1996) Analysis of quantitative inheritance for smut resistance in sugarcane. J Fujian Agric U China 25:271–275
Liu B, Xue XD, Cui SP, Zhang XY, Han QM, Zhu L, Liang XF, Wang XJ, Huang LL, Chen XM, Kang ZH (2010) Cloning and characterization of a wheat β-1,3-glucanase gene induced by the stripe rust pathogen Puccinia striiformis f. sp. tritici. Mol Biol Rep 37:1045–1052
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−△△Ct method. Methods 25:402–408
Moosawi-Jorf SA, Mahin BI (2007) In vitro detection of yeast-like and mycelial colonies of Ustilago scitaminea in tissue-cultured plantlets of sugarcane using polymerase chain reaction. J Appl Sci 7:3768–3773
Neuhaus JM, Flores SS, Keefe D, Ahl-Goy P, Meins F Jr (1992) The function of vacuolar β-1,3-glucanase investigated by antisense transformation. Susceptibility of transgenic Nicotiana sylvestris plants to Cercospora nicotianae infection. Plant Mol Biol 19:803–813
O’Kennedy MM, Crampton BG, Lorito M, Chakauya E, Breese WA, Burger JT, Botha FC (2011) Expression of a β-1,3-glucanase from a biocontrol fungus in transgenic pearl millet. S Afr J Bot 77:335–345
Padmanaban P, Alexander KC, Shanmugan N (1998) Effect of smut on growth and yield parameters of sugarcane. Indian Phytopathol 41:367–369
Paterson AH, Bowers JE, Bruggmann R, Dubchak I, Grimwood J, Gundlach H, Haberer G, Hellsten U, Mitros T, Poliakov A, Schmutz J, Spannagl M, Tang H, Wang X, Wicker T, Bharti AK, Chapman J, Feltus FA, Gowik U, Grigoriev IV, Zhou K, Lucas S, Glavinadel-Rio T, Tice H, Bruce D, Pitluck S, Lyons E, Maher CA, Martis M, Narechania A, Otillar RP, Penning BW, Salamov AA, Wang Y, Zhang L, Carpita NC, Freeling M, Gingle AR, Hash CT, Keller B, Klein P, Kresovich S, McCann MC, Ming R, Peterson DG, Mehboob UR, Ware D, Westhoff P, Mayer KFX, Messing J, Rokhsar DS (2009) The Sorghum bicolor genome and the diversification of grasses. Nature 457:551–556
Que YX, Xu LP, Xu JS, Zhang JS, Zhang MQ, Chen RK (2009a) Selection of control genes in real-time qPCR analysis of gene expression in sugarcane. Chin J Trop Crop 30:274–278
Que YX, Yang ZX, Xu LP, Chen RK (2009b) Isolation and identification of differentially expressed genes in sugarcane infected by Ustilago scitaminea. Acta Agron Sin 35:452–458
Que YX, Xu LP, Lin JW, Chen RK, Grisham MP (2012) Molecular variation of Sporisorium scitamineum in Mainland China revealed by RAPD and SRAP markers. Plant Dis 96:1519–1525
Rahaie M, Xue GP, Naghavi MR, Alizadeh H, Schenk PM (2010) A MYB gene from wheat (Triticum aestivum L.) is up-regulated during salt and drought stresses and differentially regulated between salt-tolerant and sensitive genotypes. Plant Cell Rep 29:835–844
Ramamoorthy V, Raguchander T, Samiyappan R (2002) Induction of defense-related proteins in tomato roots treated with Pseudomonas fluorescens Pf1 and Fusarium oxysporum f. sp. lycopersici. Plant Soil 239:55–68
Romero GO, Simmons C, Yaneshita M, Doan M, Thomas BR, Rodriguez RL (1998) Characterization of rice endo-β-glucanase genes (Gns2-Gns14) defines a new subgroup within the gene family. Gene 223:311–320
Roy-Choudhury S, Roy S, Singh SK, Sengupta DN (2010) Molecular characterization and differential expression of β-1,3-glucanase during ripening in banana fruit in response to ethylene, auxin, ABA, wounding, cold and light-dark cycles. Plant Cell Rep 29:813–828
Scortecci KC, Creste S, Calsa T Jr, Xavier MA, Landell MGA, Figueira A, Benedito VA (2012) Challenges, opportunities and recent advances in sugarcane breeding. In: Abdurakhmonov I (ed) Plant breeding. Nabu Press, London, pp 267–296
Stone BA, Clarke AE (1992) Chemistry and physiology of higher plant 1,3-β-glucans (callose). In: Stone BA, Clarke AE (eds) Chem biol (1,3)-β-glucans. La Trobe University Press, Bundoora, pp 365–429
Sun W, Cao ZY, Li Y, Zhao YX, Zhang H (2007) A simple and effective method for protein subcellular localization using Agrobacterium-mediated transformation of onion epidermal cells. Biologia 62:529–532
Sundar AR, Barnabas EL, Malathi P, Viswanathan R (2012) A mini-review on smut disease of sugarcane caused by Sporisorium scitamineum. In: Mworia J (ed) Botany. In Tech, Croatia, pp 109–128
Sundaresha S, Manoj Kumar A, Rohini S, Math SA, Keshamma E, Chandrashekar SC, Udayakumar M (2010) Enhanced protection against two major fungal pathogens of groundnut, Cercospora arachidicola and Aspergillus flavus in transgenic groundnut over-expressing a tobacco β-1,3-glucanase. Eur J Plant Pathol 126:497–508
Takken FLW, Joosten MHAJ (2000) Plant resistance genes: their structure, function and evolution. Eur J Plant Pathol 106:699–713
Thoakoane LN, Rutherford RS (2001) cDNA-AFLP differential display of sugarcane (saccharum spp. hybrids) genes induced by challenge with the fungal pathogen Ustilago scitaminea (sugarcane smut). Proc S Afr Sug Technol Ass 75:104–107
Van-Loon LC (1985) Pathogenesis-related proteins. Plant Mol Biol 4:111–116
Vijayendra SVN, Kashiwagi Y (2009) Characterization of a new acid stable exo-β-1,3-glucanase of Rhizoctonia solani and its action on microbial polysaccharides. Int J Biol Macromol 44:92–97
Vu JC, Allen LH Jr (2009) Growth at elevated CO2 delays the adverse effects of drought stress on leaf photosynthesis of the C4 sugarcane. J Plant Physiol 166:107–116
Wan LL, Zha WJ, Cheng XY, Liu C, Lv L, Liu CX, Wang ZQ, Du B, Chen RZ, Zhu LL, He GC (2011) A rice β-1,3-glucanase gene Osg1 is required for callose degradation in pollen development. Planta 233:309–323
Ward ER, Payne GB, Moyer MB, Williams SC, Dincher SS, Sharkey KC, Beck JJ, Taylor HT, Ahl-Goy P, Meins F Jr, Ryals JA (1991) Differential regulation of β-1,3-glucanase messenger RNAs in response to pathogen infection. Plant Physiol 96:390–397
Wu CT, Bradford KJ (2003) Class I Chitinase and β-1,3-glucanase are differentially regulated by wounding, methyl jasmonate, ethylene, and gibberellin in tomato seeds and leaves. Plant Physiol 133:263–273
Xu LP, Wang JN, Chen RK (1994) Biochemical reaction of sugarcane to smut and its relation to resistance. Sugarcane 1:13–16
Xu LP, Chen RK, Chen PH (2004) Analysis on infection index of smut caused by Ustilago scitaminea in sugarcane segregated population. Chin J Trop Crop 25:33–36
Yao W, Yu AL, Xu JS, Zhou H, Zhang MQ, Chen RK (2005) A simple and quick method for extracting sugarcane genomic DNA. Chin J Agric Biotechnol 12:121–122
Zhen XH, Li YZ (2004) Ultrastructural changes and location of β-1,3-glucanase in resistant and susceptible cotton callus cells in response to treatment with toxin of Verticillium dahliae and salicylic acid. J Plant Physiol 161:1367–1377
Acknowledgments
This work was funded by National Natural Science Foundation of China (No. 31101196), the earmarked fund for the Modern Agriculture Technology of China (CARS-20), Research Funds for Distinguished Young Scientists in Fujian Agriculture and Forestry University (xjq201202), National High Technology Research and Development Program of China (863 Program) Project (2013AA102604).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by P. Lakshmanan.
Rights and permissions
About this article
Cite this article
Su, Yc., Xu, Lp., Xue, Bt. et al. Molecular cloning and characterization of two pathogenesis-related β-1,3-glucanase genes ScGluA1 and ScGluD1 from sugarcane infected by Sporisorium scitamineum . Plant Cell Rep 32, 1503–1519 (2013). https://doi.org/10.1007/s00299-013-1463-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-013-1463-9