Abstract
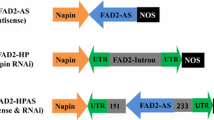
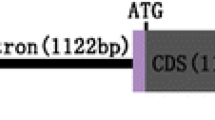
The fatty acid composition in the seed oil was significantly modified following the introduction of transgenes. To further enhance the desirable characteristics of rapeseed oil, it would be beneficial to develop a new approach for the simultaneous silencing of two or more target genes. Our goals in the current study were to (1) increase oleic acid to more than 75%, (2) reduce polyunsaturated fatty acids (PUFA) to about 10% and erucic acid to zero, and (3) accomplish these changes in a single-transformation event. In a single transformation, two fragments amplified from the fatty acid Δ12-desaturase 2 (BnaFAD2) and fatty acid elongase 1 (BnaFAE1) genes of Brassica napus were linked together to form a fusion fragment. The fusion fragment was then used to assemble unique intron-spliced hairpin interfering constructs. In the transgenic plant FFRP4-4, the expression of BnaFAD2 and BnaFAE1 genes was completely inhibited. The composition of oleic acid in FFRP4-4 rose to 85%, PUFA dropped to 10% and erucic acid was undetectable. All hybrid F1 seeds obtained from the reciprocal crossing of FFRP4-4 and GX-parents (with different genetic backgrounds) contained more than 80% oleic acid, about 10% PUFA and very low, or undetectable, erucic acid. The results confirmed that the fusion fragment silencing construct can simultaneously and effectively silence the target genes on a consistent basis. The strategy provides a useful tool for detecting gene function and advancing genetic engineering techniques for the improvement of agricultural crops.



Similar content being viewed by others
References
Ackman RG (1990) Canola fatty acids—an ideal mixture for health, nutrition and food use. In: Shahidi F (ed) Canola and rapeseed: Production, Chemistry, Nutrition, and Processing Technology. Van Nostrand Reinhold, New York, pp 81–98
Alvarez JP, Pekker I, Goldshmidt A, Blum E, Amsellem Z, Eshed Y (2006) Endogenous and synthetic microRNAs stimulate simultaneous, efficient, and localized regulation of multiple targets in diverse species. Plant Cell 18:1134–1151
Bade JB, Damm B (1995) Gene transfer to plants. In: Potrykus I, Spangenberg G (eds) Springer lab manual. Springer, Berlin, pp 30–38
Browse J, Spychalla J, Okuley J, Lightner J (1998) Altering the fatty acid composition of vegetable oils. In: Harwood JL (ed) Plant lipid biosynthesis: fundamentals and agricultural applications. Cambridge University Press, New York, pp 131–153
Burton JW, Miller JF, Vick BA, Scarth R, Holbrook CC (2004) Altering fatty acid composition in oil seed crops. Adv Agron 4:273–306
Chen W, Li JF, Dong YS, Li GZ, Cun SX, Wang JQ (2006) Obtaining new germplast of Brassica napus with high oleic acid content by RNA interference and marker-free transformation of Fad2 gene. J Plant Physiol Mol Biol 32(6):665–671
Damude HG, Kinney AJ (2008) Enhancing plant seed oils for human nutrition. Plant Physiol 147:962–968
Downey R, Craig B (1964) Genetic control of fatty acid biosynthesis in rapeseed (Brassica napus L.). J Am Oil Chem 41:475–478
Ellerström M, Stålberg K, Ezcurra I, Rask L (1996) Functional dissection of a napin gene promoter: identification of promoter elements required for embryo and endosperm-specific transcription. Plant Mol Biol 32:1019–1027
Eskin NAM, McDonald BE, Przybylski R, Malcolmson LJ, Scarth R, Mag T, Ward K, Adolph D (1996) Canola oil. In: Hui YH (ed) Edible oil and fat products: oil and oil seeds. Wiley, New York, pp 1–96
FAO (2008) Global Market Analysis. Oilseeds, oils and meals. food outlook. Available at Food and Agriculture Organization of the United Nations. http://www.fao.org/docrep/011/ai474e/ai474e07.htm
Finnegan J, McElroy D (1994) Transgene inactivation: plant fight back. Nat Biotechnol 12:883–888
Gutiérrez-Nava ML, Aukerman MJ, Sakai H, Tingey SV, Williams RW (2008) Artificial trans-acting siRNAs confer consistent and effective gene silencing. Plant Physiol 147:543–551
Hardin-Fanning F (2008) The effects of a Mediterranean-style dietary pattern on cardiovascular disease risk. Nurs Clin North Am 43:105–115
Harvey B, Downey R (1963) The inheritance of erucic acid content in rapeseed (Brassica napus L.). Can J Plant Sci 44:104–111
James DW, Lim E, Keller J, Plooy I, Ralston E, Dooner HK (1995) Directed tagging of the Arabidopsis fatty acid elongation1 (FAE1) gene with the maize transposon activator. Plant Cell 7:309–319
Kinney AJ (1994) Genetic modification of the storage lipids of plants. Curr Opin Biotechnol 5:144–151
Knutzon DS, Thompson GA, Radke SE, Johnson WB, Knauf VC, Kridl JC (1992) Modification of Brassica seed oil by antisense expression of a stearoyl-acyl carrier protein desaturase gene. Proc Natl Acad Sci USA 89:2624–2628
Lauridsen C, Nielsen JH, Henckel P, Sørensen MT (1999) Antioxidative and oxidative status in muscles of pigs fed on rapeseed oil, vitamin E and copper. J Anim Sci 77:105–115
Liu Q, Singh SP, Green AG (2002) High-stearic and high-oleic cottonseed oils produced by hairpin RNA-mediated post-transcriptional gene silencing. Plant Physiol 129:1732–1743
McVetty PBE, Scarth R (2002) Breeding for improved oil quality in Brassica oilseed species. J Crop Prod 5(1):345–369
Misra S (1990) Transformation of Brassica napus L. with a ‘Disarmed’ octopine plasmid of Agrobacterium tumefaciens: molecular analysis and inheritance of the transformed phenotype. J Exp Bot 41:269–275
Moloney MM, Walker JM, Sharma KK (1989) High efficiency transformation of Brassica napus using agrobacterium vectors. Plant Cell Rep 8(4):238–242
Østergaard L, King G (2008) Standardized gene nomenclature for the Brassica genus. Plant Methods 4:10
Peerbolte R, Leenhouts K, Hooykass-van Slogteren GMS, Wullems GJ (1986) Clone from a shoort tobacco crown gall tumorll: irregular T-DNA structures and organization T-DNA, methylation and conditional expression of opine genes. Plant Mol Biol 7:285–299
Przybylski R (2005) Canola oil:physical and chemical properties. Available at Canola Council of Canada. https://canola-council.merchantsecure.com/canola_resources/product43.aspx
Radke SE, Andrews BM, Moloney MM, Crouch ML, Kridl JC, Knauf VC (1988) Transformation of Brassica napus L. using Agrobacterium tumefaciens: developmentally regulated expression of a reintroduced napin gene. Theor Appl Genet 75:685–694
Röbbelen G, Nitsch A (1975) Genetical and physiological investigations on mutants for polyenoic fatty acids in rapeseed, B. napus L. I. Selection and description of new mutants. Z Pflanzenzüchtg 75:93–105
Rücker B, Röbbelen G (1995) Development of high oleic acid winter rapeseed. In: Proceedings of 9th international rapeseed congress, Cambridge, UK, pp 389–391
Scarth R (1995) Developments in the breeding of edible oil in Brassica napus and B. rapa. In: Proceedings of 9th international rapeseed congress: rapeseed today and tomorrow, Cambridge, UK, pp 377–382
Scarth R, Tang JH (2006) Modification of Brassica oil using conventional and transgenic approaches. Crop Sci 46:1225–1236
Scarth R, McVetty PBE, Rimmer SR, Stefansson BR (1988) Stellar low linolenic-high linoleic acid summer rape. Can J Plant Sci 68:509–511
Schierholt A, Rücker B, Becker HC (2001) Inheritance of high oleic acid mutations in winter oilseed rape (Brassica napus L.). Crop Sci 41:1444–1449
Schwab R, Ossowski S, Riester M, Warthmann N, Weigel D (2006) Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell 18:1121–1133
Shahidi F (1990) Rapeseed and canola: global production and distribution. In: Shahidi F (ed) Canola and rapeseed: Production, Chemistry, Nutrition, and Processing Technology. Van Nostrand Reinhold, New York, pp 3–13
Sivaraman I, Arumugam N, Sodhi YS, Gupta V, Mukhopadhyay A, Pradhan AK, Burma PK, Pental D (2004) Development of high oleic and low linoleic acid transgenics in a zero erucic acid Brassica juncea L. (Indian mustard) line by antisense suppression of the fad2 gene. Mol Breed 13:365–375
Smith MA, Moon H, Chowrira G, Kunst L (2003) Heterologous expression of a fatty acid hydroxylase gene in developing seeds of Arabidopsis. Planta 217:507–516
Sovero M (1993) Rapeseed, a new oilseed crop for the United States. In: Janick J, Simon JE (eds) New crops. Wiley, New York, pp 302–307
Stålberg K, Ellerström M, Josefsson LG, Rask L (1993) Deletion analysis of a 2S seed storage protein promoter of Brassica napus in transgenic tobacco. Plant Mol Biol 23:671–683
Stefansson BR, Downey RK (1995) Rapeseed. In: Slinkard AE, Knott DR (eds) Harvest of gold: the history of field crop breeding in Canada. University Extension Press, University of Saskatchewan, Saskatoon, pp 140–152
Stoutjesdijk PA, Hurlestone C, Singh SP, Green AG (2000) High-oleic acid Australian Brassica napus and B. juncea varieties produced by co-suppression of endogenous D12–desaturases. Biochem Soc Trans 28:938–940
Stoutjesdijk PA, Singh SP, Liu, Hurlstone CJ, Waterhouse PA, Green AG (2002) hpRNA-mediated targeting of the Arabidopsis FAD2 gene gives highly efficient and stable silencing. Plant Physiol 129:1723–1731
Taylor DC, MacKenzie SL, McCurdy AR, McVetty PBE, Giblin EM, Pass EW, Stone SJ, Scarth R, Rimmer SR, Pickard MD (1994) Stereospecific analyses of seed triacylglycerols from high-erucic acid Brassicaceae: detection of erucic acid at the sn-2 position in Brassica oleracea L. genotypes. J Am Oil Chem Soc 71:163–167
Töpfer R, Martini N, Schell J (1995) Modification of plant lipid synthesis. Science 268:681–686
Vilkki JP, Tanhuanpää PK (1995) Breeding of high oleic acid spring turnip rape in Finland. In: Proceedings of 9th international rapeseed congress, Cambridge, UK, pp 386–388
Warner K, Mounts TL (1993) Frying stability of soybean and canola oils with modified fatty acid compositions. J Am Oil Chem Soc 60:983–988
Wong R, Patel JD, Grant I, Parker J, Charne D, Elhalwagy M, Sys E (1991) The development of high oleic canola. In: Abstracts, 8th international rapeseed congress, Saskatoon, Canada, pp 53
Wu G, Wu YH, Xiao L, Li XD, Lu CM (2008) Zero erucic acid trait of rapeseed (Brassica napus L.) results from a deletion of four base pairs in the fatty acid elongase 1 gene. Theor Appl Genet 116:491–499
Zhang HJ, Xiao G, Tan TL, Li X, Guan CY (2008) High oleate material of rapeseed (Brassica napus) produced by EMS treatment. Sci Agric Sin 41(12):4016–4022
Acknowledgments
This work was financially supported by the National High Technology and Development Program of China (2008AA10Z152), and by the Development Plan of the State Key Fundamental Research of China (2006CB101603).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by R. Schmidt.
Rights and permissions
About this article
Cite this article
Peng, Q., Hu, Y., Wei, R. et al. Simultaneous silencing of FAD2 and FAE1 genes affects both oleic acid and erucic acid contents in Brassica napus seeds. Plant Cell Rep 29, 317–325 (2010). https://doi.org/10.1007/s00299-010-0823-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-010-0823-y