Abstract
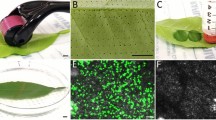
Agrobacterium-mediated transient assays for the analysis of gene function are used as alternatives to genetic complementation and stable plant transformation. Although such assays are routinely performed in several plant species, they have not yet been successfully applied to grapevines. We explored genetic background diversity of grapevine cultivars and performed agroinfiltration into in vitro cultured plants. By combining different genotypes and physiological conditions, we developed a protocol for efficient transient transformations of selected grapevine cultivars. Among the four cultivars analyzed, Sugraone and Aleatico exhibited high levels of transient transformation. Transient expression occurred in the majority of cells within the infiltrated tissue several days after agroinfiltration and, in a few cases, it later spread to a larger portion of the leaf. Three laboratory strains of Agrobacterium tumefaciens with different virulence levels were used for agroinfiltration assays on grapevine plants. This method promises to be a powerful tool to perform subcellular localization analyses. Grapevine leaf tissues were transformed with fluorescent markers targeted to cytoplasm (free GFP and mRFP1), endoplasmatic reticulum (GFP::HDEL), chloroplast (GAPA1::YFP) and mitochondria (β::GFP). Confocal microscope analyses demonstrated that these subcellular compartments could be easily visualized in grapevine leaf cells. In addition, from leaves of the Sugraone cultivar agroinfiltrated with endoplasmic reticulum-targeted GFP-construct, stable transformed cells were obtained that show the opportunity to convert a transiently transformed leaf tissue into a stably transformed cell line.






Similar content being viewed by others
References
Batoko H, Zheng HQ, Hawes C, Moore I (2000) A Rab1 GTPase is required for transport between the endoplasmic reticulum and Golgi apparatus and for normal Golgi movement in plants. Plant Cell 12:2201–2217
Brandizzi F, Fricker M, Hawes C (2002) A greener world: the revolution in plant bioimaging. Nat Rev Mol Cell Biol 3:520–530
Campbell RE, Tour O, Palmer AE, Steinbach PA, Baird GS, Zacharias DA, Tsien RY (2002) A monomeric red fluorescent protein. Proc Natl Acad Sci USA 99:7877–7882
Carimi F, Barizza E, Gardiman G, Lo Schiavo F (2005) Somatic embryogenesis from stigmas and styles of grapevine. In Vitro Cell Dev Biol 41:249–252
Chee R, Pool RM (1987) Improved inorganic media constituents for in vitro shoot moltiplication of Vitis. Sci Hortic 32:85–95
Citovsky V, Kozlovsky SV, Lacroix B, Zaltsman A, Dafny-Yelin M, Vyas S, Tovkach A, Tzfira T (2007) Biological systems of the host cell involved in Agrobacterium infection. Cell Microbiol 9: 9–20
daSilva LL, Snapp EL, Denecke J, Lippincott-Schwartz J, Hawes C, Brandizzi F (2004) Endoplasmic reticulum export sites and Golgi bodies behave as single mobile secretory units in plant cells. Plant Cell 16:1753–1771
Duby G, Oufattole M, Boutry M (2001) Hydrophobic residues within the predicted N-terminal amphiphilic alpha-helix of a plant mitochondrial targeting presequence play a major role in in vivo import. Plant J 27:539–549
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Hamilton RH, Fall MZ (1971) The loss of tumor-initiating ability in Agrobacterium tumefaciens by incubation at high temperature. Experientia 27:229–230
Haseloff J, Siemering KR, Prasher DC, Hodge S (1997) Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc Natl Acad Sci USA 94:2122–2127
Hellens RP, Edwards EA, Leyland NR, Bean S, Mullineaux PM (2000a) pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol Biol 42:819–832
Hellens RP, Mullineaux PM, Klee H (2000b) A guide to Agrobacterium binary Ti vectors. Trends Plant Sci 5:446–451
Hoekema A, Hirsch PR, Hooykaas PJJ, Schilperoort RA (1983) A binary plant vector strategy based on separation of vir- and T-region of the Agrobacterium tumefaciens Ti-plasmid. Nature 303:179–180
Jones HD, Doherty A, Wu H (2005) Review of methodologies and a protocol for the Agrobacterium-mediated transformation of wheat. Plant Methods 5:1–9
Kapila J, De Rycke R, Van Montagu M, Angenon G (1997) An Agrobacterium-mediated transient gene expression system for intact leaves. Plant Sci 122:101–108
Latz A, Ivashikina N, Fischer S, Ache P, Sano T, Becker D, Deeken R, Hedrich R (2007) In planta AKT2 subunits constitute a pH- and Ca2+-sensitive inward rectifying K+ channel. Planta 225:1179–1191
Main GD, Reynolds S, Gartland JS (1995) Electroporation protocols in Agrobacterium. In: Gartland KMA, Davey MR (eds) Methods in molecular biology, vol 44: Agrobacterium protocols. Humana Press, Totowa, pp 405–412
Marri L, Trost P, Pupillo P, Sparla F (2005) Reconstitution and properties of the recombinant glyceraldehyde-3-phosphate dehydrogenase/CP12/phosphoribulokinase supramolecular complex of Arabidopsis. Plant Physiol 139:1433–1443
Melotto M, Underwood W, Koczan J, Nomura K, He SY (2006) Plant stomata function in innate immunity against bacterial invasion. Cell 126:969–980
Mezzetti B, Pandolfini T, Navacchi O, Landi L (2002) Genetic transformation of Vitis vinifera via organogenesis. BMC Biotechnol 27:2–18
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Schöb H, Kunz C, Meins F Jr (1997) Silencing of transgenes introduced into leaves by agroinfiltration: a simple, rapid method for investigating sequence requirements for gene silencing. Mol Gen Genet 256:581–585
Taiz L, Zeiger E (2006) Plant physiology, 4th edn. Sinauer Associates, Sunderland
The French–Italian Public Consortium for Grapevine Genome Characterization (2007) The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449:463–467
von Arnim AG, Deng XW, Stacey MG (1998) Cloning vectors for the expression of green fluorescent protein fusion proteins in transgenic plants. Gene 221:35–43
Wroblewski T, Tomczak A, Michelmore R (2005) Optimization of Agrobacterium-mediated transient assays of gene expression in lettuce, tomato and Arabidopsis. Plant Biotechnol J 3:259–73
Yang Y, Li R, Qi M (2000) In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant J 22:543–551
Zhao R, Dielen V, Kinet JM, Boutry M (2000) Cosuppression of a plasma membrane H+-ATPase isoform impairs sucrose translocation, stomatal opening, plant growth, and male fertility. Plant Cell 12:535–546
Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, Boller T, Felix G (2006) Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125:749–760
Acknowledgments
We thank Professor Roger Tsien for providing us the pRSET-mRFP1 vector and Prof. Albrecht von Arnim for providing the pAVA554-YFP vector. We thank Dr. Jim Haseloff for providing the pBIN-m-gfp5-ER vector. We thank Prof. Paolo Trost for providing the GAPA1 cDNA clone. This research was supported by the “Progetto di Ateneo” by the University of Padova to M. Z.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. Somers.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zottini, M., Barizza, E., Costa, A. et al. Agroinfiltration of grapevine leaves for fast transient assays of gene expression and for long-term production of stable transformed cells. Plant Cell Rep 27, 845–853 (2008). https://doi.org/10.1007/s00299-008-0510-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-008-0510-4