Abstract
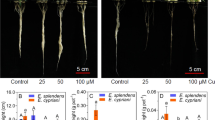
Roots of Panax ginseng exposed to various concentrations of Cu (0.0, 5, 10.0, 25.0, and 50.0 μM) accumulated high amounts of Cu in a concentration-dependent and duration-dependent manner. Roots treated with 50 μM Cu resulted in 52% and 89% growth inhibition after 20 and 40 days, respectively. Saponin synthesis was stimulated at a Cu concentration between 5 and 25 μM but decreased at 50 μM Cu. Malondialdehyde content (MDA), lipoxygenase activity (LOX), superoxide ion (O2 •−) accumulation, and H2O2 content at 5 and 10 μM Cu-treated roots were not increased but strongly increased at 50 μM Cu resulting in the oxidation of ascorbate (ASC) and glutathione (GSH) to dehydroascorbate (DHA) and glutathione disulfide (GSSG), respectively indicating a clear oxidative stress. Seven well-resolved bands of superoxide dismutase (SOD) were detected in the gel and an increase in SOD activity seemed to be mainly due to the induction of Fe-SOD 3. Five to 10 μM Cu slightly induced activity of ascorbate peroxidase (APX) and dehydroascorbate reductase (DHAR), guaiacol peroxidase (G-POD) but inhibited monodehydroascorbate reductase (MDHAR) and glutathione reductase (GR) enzyme activities. No changes in catalase (CAT) activity and in activity gel were found up to 25 μM Cu, but both G-POD and CAT activities were inhibited at 50 μM Cu. Glutathione metabolism enzymes such as γ-glutamylcysteine synthetase (γ-GCS), glutathione-S-transferase (GST), and glutathione peroxidase activities (GPx) were activated at 5 and 10 μM Cu but were strongly inhibited at 50 μM Cu due to the Cu accumulation in root tissues. The strong depletion of GSH at 50 μM Cu was associated to the strong induction of γ-glutamyltranspeptidase (γ-GGT) activity. These results indicate that plant could grow under Cu stress (5–25 μM) by modulating the antioxidant defense mechanism for combating Cu induced oxidative stress.






Similar content being viewed by others
Abbreviations
- APX:
-
Ascorbate peroxidase (EC 1.11.1.11)
- CAT:
-
Catalase (EC 1.11.1.6)
- CBN:
-
Chungbuk National University Line 1
- DHAR:
-
Dehydroascorbate reductase (EC 1.8.5.1)
- DHA:
-
Dehydroascorbate
- GST:
-
Glutathione-S-transferase (EC 2.5.1.18)
- GPx:
-
Glutathione peroxidase (EC 1.11.1.12)
- GR:
-
Glutathione reductase (EC 1.6.4.2)
- G-POD:
-
Guaiacol peroxidase (EC 1.11.1.7)
- MDA:
-
Malondialdehyde
- MDHAR:
-
Monodehydroascorbate reductase (EC 1.6.5.4)
- NBT:
-
Nitroblue tetrazolium
- ROS:
-
Reactive oxygen species
- ASC:
-
Reduced ascorbate
- SOD:
-
Superoxide dismutase (EC 1.15.1.1)
- XTT:
-
Sodium,3,-[1-[phenylamino-carbonyl]-3,4-tetrazolium]-bis(4-methoxy-6-nitro)benzene-sulfonic acid hydrate
References
Able AJ, Guest DI, Sutherland MW (1998) Use of a new tetrazolium-based assay to study the production of superoxide radicals by tobacco cell cultures challenged with avirulent zoospores of Phytophthora parasitica var nicotianae. Plant Physiol 117:491–499
Aebi H (1984) Catalases. In: Bergmeyer HU (ed) Methods of enzymatic analysis 2. Academic Press, New York, pp 673–684
Alaoui-Sossé B, Genet P, Vinit-Dunand F, Toussaint ML, Epron D, Badot PM (2004) Effect of copper on growth in cucumber plants (Cucumis sativus) and its relationships with carbohydrate accumulation and changes in ion contents. Plant Sci 166:1213–1218
Alexieva V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ 24:1337–1344
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1331–1341
Ashraf M, Harris PJC (2004) Potential biochemical indicators of salinity tolerance in plants. Plant Sci 166:3–16
Attele AS, Wu JA, Yuan CS (1999) Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol 58:1685–1693
Axerold B, Chesbrough TM, Laakso S (1981) Lipoxygenase from soybean. In: Lowenstein JM (ed) Methods enzymology. Academic Press, New York, pp 441–451
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in condition. Anal Biochem 161:559–566
Bradford MM (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen GX, Asada K (1989) Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol 30:987–998
Chen XC, Zhu YG, Zhu LA, Huang C, Chen Y, Chen LM, Fang F, Zhou YC, Zhao CH (2003) Ginsenoside Rg1 attenuates dopamine-induced apoptosis in PC12 cells by suppressing oxidative stress. Eur J Pharmacol 473:1–7
Doulis AG, Debian N, Kingston-Smith AH, Foyer CH (1997) Differential localization of antioxidants in maize. Plant Physiol 114:1031–1037
Drạżkiewicz M, PSkórzyńska-olit E, Krupa Z (2003) Response of the ascorbate–glutathione cycle to excess copper in Arabidopsis thaliana (L.). Plant Sci 164:195–202
Droter A, Phclps P, Fall R (1985) Evidence for glutathione peroxidase activities in cultured plant cells. Plant Sci 42:35–40
Ferrat L, Barelli MG, Martini CP, Romeo M (2003) Mercury and non-protein thiol compounds in the seagrass Posidonia oceanica. Comp Biochem Physiol 134:147–155
Fielding JL, Hall JL (1978) A biochemical and cytological study of peroxidase activity in roots of Pisum sativum. J Exp Bot 29:969–981
Gaetke LM, Chow CK (2003) Copper toxicity, oxidative stress and antioxidant nutrients. Toxicology 189:147–163
Gara LD (2003) Ascorbate metabolisms and plant growth: from germination to cell death. In: Asard H, Smirnoff J and May N (eds) Vitamin C: its function and biochemistry in animals and plants, Bios Scientific Publisher, Oxford, pp 83–95
Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106:207–211
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hodenberg A, Fink A (1975) Ermittlung von Toxizitäts-Grenzwerten für Zink, Kupfer und Blei in Hafer und Rotklee. Z. Planzenernähr Bodenk 138:489–503
Hossain MA, Nakano Y, Asada K (1984) Monodehydroascorbate reductase in spinach chloroplasts and its participation in regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol 25:385–395
Kim JH, Kim S, Yoon IS, Lee JH, Jang BJ, Jeong SM, Lee JH, Lee BHHJ, Oh S (2005) Protective effects of ginseng saponins on 3-nitropropionic acid-induced striatal degeneration in rats. Neuropharmacology 48:743–756
Konno H, Nakato T, Nakashima S, Katoh K (2005) Lygodium japonicum fern accumulates copper in the cell wall pectin. J Exp Bot 56:1923–1931
Kretchmer OA, Dahan YG, Yadun SL, Gollop R, Hayyim GB (2004) The salt-stress signal transduction pathway that activates the gpx1 promoter is mediated by intracellular H2O2, different from the pathway induced by extracellular H2O2. Plant Physiol 135:1685–1696
Kubo A, Aono M, Nakajima N, Saji H, Tanaka K, Kondo N (1999) Differential responses in activity of antioxidant enzymes to different environmental stresses in Arabidopsis thaliana. J Plant Res 112:279–290
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lee EJ, Ko E, Lee J, Rho S, Ko S, Shin MK, Min B, Hong MC, Kim SY, Bae H (2004) Ginsenoside Rg1 enhances CD4+ T-cell activities and modulates Th1/Th2 differentiation. Int Immunopharmacol 4:235–244
Mazhoudi S, Chaoui A, Ghorbal MH, El Ferjani E (1997) Response of antioxidant enzymes to excess copper in tomato (Lycopersicon esculentum, Mill.). Plant Sci 127:129–137
Marschner H (1995) Mineral nutrition of higher plants, vol II. Academic Press, London, pp. 337–347
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 9:405–410
Montillet JL, Cacas JL, Garnier L, Montané MH, Douki T, Bessoule JJ, Kowalczyk LP, Maciejewska U, Agnel JP, Vial A, Triantaphylidès C (2004) The upstream oxylipin profile of Arabidopsis thaliana: a tool to scan for oxidative stresses. Plant J 40:439–451
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue. Physiol Plant 15:473–497
Nagalakshmi N, Prasad MNV (2001) Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci 160:291–299
Neill SJ, Desikan R, Clarke A, Hurst RD, Hancock JT (2002a) Hydrogen peroxide and nitric oxide as signalling molecules in plants. J Exp Bot 53:1237–1242
Neill SJ, Desikan R, Hancock JT (2002b) Hydrogen peroxide signaling. Curr Opi Plant Biol 5:388–395
Noctor G, Gomez L, Vanacker H, Foyer CH (2002) Interactions between biosynthesis, compartmentation, and transport in the control of glutathione homeostasis and signaling. J Exp Bot 53:1283–1304
Noctor G, Strohm M, Jouanin L, Kunert KJ, Foyer CH, Rennenberg H (1996) Synthesis of glutathione in leaves of transgenic poplar overexpressing γ-glutamylcysteine synthetase. Plant Physiol 112:1071–1078
Pagila DE, Valentine WN (1969) Valentine studies on the quantitative and qualitative characterization of erythrocytes glutathione peroxidase. J Lab Clin Med 70:158–169
Prasad KVSK, Pardha SP, Sharmila P (1999) Concerted action of antioxidant enzymes and curtailed growth under zinc toxicity in Brassica juncea. Environ Exp Bot 42:1–10
Pütter J (1971) Peroxidases. In: Bergmeyer HU (ed) Methods of enzymatic analysis 2. Academic Press, New York, pp 685–690
Rai V, Kakkar P, Khatoon S, Rawat AKS, Mehrotra S (2001) Heavy metal accumulation in some herbal drugs. Pharm Biol 39:384–387
Ražić S, Onjia A, Đogo S, Slavković L, Popović A (2005) Determination of metal content in some herbal drugs—Empirical and chemometric approach. Talanta 67:233–239
Robson AD, Reuter DJ (1981) Diagnosis of copper deficiency and toxicity. In: Loneragan JF, Robson AD, Graham RD (eds) Copper in soils and plants, Academic Press, London, pp 287–312
Roxas VP, Lodhi SA, Garrett DK, Mahan JR, Allen RD (2000) Stress tolerance in transgenic tobacco seedlings that overexpress glutathione S-transferase/glutathione peroxidase. Plant Cell Physiol 41:1229–1234
Rutenberg MA, Kim IT, Eischbein JW, Hanker JS, Wesservkrug HL, Seligman AM (1969) Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem 17:517
Seeling GF, Meister A (1984) γ-Glutamylcysteine synthetase. J Biol Chem 259:3534–3538
Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, Yoshimura K (2002) Regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot 53:1305–1319
Smith IK, Vierheller TL, Thorne CA (1988) Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis (2-nitrobenzoic acid). Anal Biochem 175:408–413
Smith IK, Vierheller TL, Thorne CA (1989) Properties and functions of glutathione reductase in plants. Physiol Plant 77:449–456
Storozhenko S, Boix EB, Babiychuk E, Hérouart D, Davey MW, Slooten L, Montagu MV, Inzé D, Kushnir S (2002) γ-glutamyl transpeptidase in transgenic tobacco plants. cellular localization, processing, and biochemical properties. Plant Physiol 128:1109–1119
Takahama U, Oniki T (1992) Regulation of peroxidase-dependent oxidation of phenolics in the apoplast of spinach leaves by ascorbate. Plant Cell Physiol 33:279–387
Woodbury W, Spencer AK, Stahmann MA (1971) An improved procedure using ferricyanide for detecting catalase isozymes. Anal Biochem 44:301–305
Yu KW, Gao WY, Hahn EJ, Paek KY (2002) Jasmonic acid improves ginsenoside accumulation in adventitious root culture of Panax ginseng C.A. Meyer. Biochem Eng J 11:211–215
Zhao J, Davis LC, Verpoorte R (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Review article. Biotechnol Advances 23:283–333
Zheljazkov VD, Nielsen NE (1996a) Studies on the effect of heavy metals (Cd, Pb, Cu, Mn, Zn and Fe) upon the growth, productivity and quality of Lavender (Lavandula angustifolia Mill.). J Essent Oil Res 8:259–274
Zheljazkov VD, Nielsen NE (1996b) Effect of heavy metals on peppermint and cornmint. Plant Soil 178:59–66
Acknowledgements
This work was financially supported by the Ministry of Education and Human Resources Development (MOE), the Ministry of Commerce, Industry and Energy (MOCIE) and the Ministry of Labour (MOLAB), Republic of Korea through the fostering project of the laboratory of Excellency. One of the authors (MBA) also wishes to acknowledge the Japanese Society for the Promotion of Science (JSPS) for providing financial assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Van Onckelen
Rights and permissions
About this article
Cite this article
Ali, M.B., Hahn, EJ. & Paek, KY. Copper-induced changes in the growth, oxidative metabolism, and saponin production in suspension culture roots of Panax ginseng in bioreactors. Plant Cell Rep 25, 1122–1132 (2006). https://doi.org/10.1007/s00299-006-0174-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-006-0174-x