Abstract
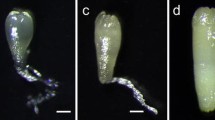
The combination of abscisic acid (ABA) and activated carbon increased Norway spruce (Picea abies L., Karst.) cotyledonary somatic embryo yields, increased the number of genotypes forming cotyledonary embryos, caused embryos to form that exhibited improved maturation characteristics, and reduced embryo production costs. Somatic embryos increased in size, showed larger apical regions, became more zygotic-like in shape, and showed higher percentages of epicotyl development upon germination. Analyses of medium for free ABA in the presence of activated charcoal showed a rapid decrease within a few hours followed by a gradual decline over the next few days with little change from 2 to 6 weeks. Gelling agents strongly affected ABA adsorption, with agar decreasing the adsorption of ABA compared to gellan gum (Gelrite, Phytagel). Over 4,000 somatic seedlings from 20 clones were produced and established in a greenhouse using the methods discussed, and approximately 1,250 seedlings representing seven clones were established in a field setting.




Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- AC:
-
Activated carbon
- BA:
-
6-Benzylaminopurine
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- ESM:
-
Embryo suspensor mass
- NAA:
-
α-Naphthalene acetic acid
References
Becwar MR, Wann SR, Nagmani R (1987) A method for quantification of the level of somatic embryogenesis among Norway spruce callus lines. Plant Cell Rep 6:35-38
Brenner ML (1981) Modern methods for plant growth substance analysis. Annu Rev Plant Physiol 32:511–538
Buchheim JA, Colburn SM, Ranch JP (1989) Maturation of soybean somatic embryos and the transition to plantlet growth. Plant Physiol 89:768–775
Capuana M, Debergh PC (1997) Improvement of the maturation and germination of horse chestnut somatic embryos. Plant Cell Tissue Organ Cult 48:23–29
Chee PP (1996) Plant regeneration from somatic embryos of Taxus brevifolia. Plant Cell Rep 16:184–187
Constantin MJ, Henke RR, Mansur MA (1977) Effect of activated charcoal on callus growth and shoot organogenesis in tobacco. In Vitro 5:293–296
Dunstan DI, Dong JZ, Carrier DJ, Abrams SR (1998) Events following ABA treatment of spruce somatic embryos. In Vitro Cell Dev Biol Plant 34:159–168
Ebert A, Taylor HF (1990) Assessment of the changes of 2,4-dichlorophenoxyacetic acid concentrations in plant tissue culture media in the presence of activated charcoal. Plant Cell Tissue Organ Cult 20:165–172
Ebert A, Taylor F, Blake J (1993) Changes of 6-benzylaminopurine and 2,4-dichlorophenoxyacetic acid concentrations in plant tissue culture media in the presence of activated charcoal. Plant Cell Tissue Organ Cult 33:157–162
Fridborg G, Landstrom L, Eriksson T (1978) The effect of activated charcoal on tissue cultures: adsorption of metabolites inhibiting morphogenesis. Physiol Plant 43:104–106
Gupta PK, Durzan DJ (1986) Plantlet regeneration via somatic embryogenesis from subcultured callus of mature embryos of Picea abies (Norway spruce). In Vitro Cell Dev Biol 22:685–688
Gupta PK, Pullman GS (1991) Method for reproducing coniferous plants by somatic embryogenesis using abscisic acid and osmotic potential variation. US Patent 5036007
Gupta PK, Timmis R, Pullman GS, Yancey M, Kreitinger M, Carlson W, Carpenter C (1991) Development of an embryogenic system for automated propagation of forest trees. In: Vasil IK (ed) Scale-up and automation in plant propagation. Academic, New York, pp 76–90
Gupta PK, Pullman G, Timmis R, Kreitinger M, Carlson W, Grob J, Welty E (1993) Forestry in the 21st century: the biotechnology of somatic embryogenesis. Biotechnology 11:454–459
Gupta PK, Grob JA (1995) Somatic embryogenesis in conifers. In: Jain S, Gupta P, Newton R (eds) Somatic embryogenesis in woody plants. Kluwer, Dordrecht, pp 81–98
Johansson L, Barbro A, Eriksson T (1982) Improvement of anther culture technique: activated charcoal bound in agar medium in combination with liquid medium and elevated CO2 concentration. Physiol Plant 54:24–30
Johansson L (1983) Effects of activated carbon in anther cultures. Physiol Plant 59:397–403
Johannson L, Calleberg E, Gedin A (1990) Correlations between activated carbon, Fe-EDTA and other organic media ingredients in cultured anthers of Anemone canadensis. Physiol Plant 80:243–249
Kapik RH, Dinus RJ, Dean JF (1995) Abscisic acid and zygotic embryogenesis in Pinus taeda. Tree Physiol 15:405–409
Li XY, Huang H, Gbur EE Jr (1997) Polyethylene glycol-promoted development of somatic embryos in loblolly pine (Pinus taeda L.). In Vitro Cell Dev Biol Plant 33:184–189
Li XY, Huang H, Murphy BJ, Gbur EE Jr (1998) Polyethylene glycol and maltose enhance somatic embryo maturation in loblolly pine (Pinus taeda L.). In Vitro Cell Dev Biol Plant 34:22–26
Neill SJ, Horgan R (1987) Abscisic acid and related compounds. In: Rivier L, Crozier A (eds) Principles and practice of plant hormone analysis, vol 1. Academic, London, UK, pp 111–167
Nissen SJ, Sutter EG (1990) Stability of IAA and IBA in nutrient medium to several tissue culture procedures. HortScience 25:800–802
Parry AD, Horgan R (1991) Physico-chemical methods in ABA research. In: Davies WJ, Jones HG (eds) Abscisic Acid. Bios, Oxford, pp 5–22
Pan JJ, van Staden J (1998) The use of charcoal in in vitro culture—a review. Plant Growth Regul 26:155–163
Pullman GS, Gupta PK (1991) Method for reproducing coniferous plants by somatic embryogenesis using adsorbent materials in the development stage. US Patent 5034326
Pullman GS, Gupta PK (1994) Method for reproducing conifers by somatic embryogenesis using mixed growth hormones for embryo culture. US Patent 5294549. Issued 15 March 1994
Stasolla C, Yeung EC (2003) Recent advances in conifer somatic embryogenesis: improving somatic embryo quality. Plant Cell Tissue Organ Cult 74:15–35
Stasolla C, Kong L, Yeung EC, Thorp TA (2002) Maturation of somatic embryos in conifers: morphogenesis, physiology, biochemistry and molecular biology. In Vitro Cell Dev Biol Plant 38:93–105
Tautorus TE, Fowke LC, Dunstan DI (1991) Somatic embryogenesis in conifers. Can J Bot 69:1873–1899
Weatherhead MA, Burdon J, Henshaw GG (1978) Some effects of activated charcoal as an additive to plant tissue culture media. Z Pflanzenphysiol 89:141–147
Van Winkle S (2000) The effect of activated carbon on the organic and elemental composition of plant tissue culture medium. PhD Thesis, Institute of Paper Science and Technology, Atlanta, Ga.
Van Winkle S, Johnson S, Pullman GS (2003) The impact of gelrite and activated carbon on the elemental composition of plant tissue culture media. Plant Cell Rep 21:1175–1182
Von Aderkas P, Label P, Lelu MA (2002) Charcoal affects early development and hormonal concentrations of somatic embryos of hybrid larch. Tree Physiol 22:431–434
Acknowledgements
The authors gratefully acknowledge the help of Hoang Phan, Doris Budworth, and Carol Schofield in carrying out media development experiments, Keith Gehr for assistance in the manuscript preparation, Mayumi Ikeda for ABA-AC analyses, and Karen Messer, Pat Carlton, Paul Forsberg, and Edith Morrow for assistance in somatic embryo germination
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Debergh
Rights and permissions
About this article
Cite this article
Pullman, G.S., Gupta, P.K., Timmis, R. et al. Improved Norway spruce somatic embryo development through the use of abscisic acid combined with activated carbon. Plant Cell Rep 24, 271–279 (2005). https://doi.org/10.1007/s00299-005-0933-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-005-0933-0