Abstract
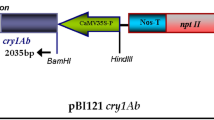
Transgenic radiata pine (Pinus radiata D. Don) plants containing a Bacillus thuringiensis (Bt) toxin gene, crylAc, were produced by means of biolistic transformation of embryogenic tissue. Using the selectable marker gene nptII and corresponding geneticin selection, 20 independent transgenic lines from five genotypes were established. Over 200 plants regenerated from ten transgenic lines were successfully transferred to soil. The integration and expression of the introduced genes in transgenic tissue and/or plants were confirmed by PCR, Southern hybridisation and neomycin phosphotransferase II (NPTII) and Bt ELISA assays. Bioassays with larvae of the painted apple moth, Teia anartoides, demonstrated that transgenic plants displayed variable levels of resistance to insect damage, with one transgenic line being highly resistant to feeding damage.






Similar content being viewed by others
Abbreviations
- ELISA :
-
Enzyme-linked immunosorbent assay
- nptII/NPTII :
-
Neomycin phosphotransferase gene or protein
- uidA/GUS :
-
β-Glucuronidase gene or protein
References
Adamczyk JJ Jr, Sumerford DV (2001) Potential factors impacting season-long expression of Cry1Ac in 13 commercial varieties of Bollgard cotton. J Insect Sci 1:1–6
Adamczyk JJ Jr, Hardee DD, Adams LC, Sumerford DV (2001) Correlating differences in larval survival and development of bollworms (Lepidoptera: Noctuidae) and fall armyworms (Lepidoptera: Noctuidae) to differential expression of CrylAc δ-endotoxin in various plant parts among commercial cultivars of transgenic Bacillus thuringiensis cotton. J Econ Entomol 94:284–290
Beck E, Ludwig G, Auerswald E, Reiss B, Schaller H (1982) Nucleotide sequence and exact localization of the neomycin phosphotransferase gene from transposon Tn5. Gene 19:327–336
Bishop-Hurley SL, Zabkiewicz RJ, Grace L, Gardner RC, Wagner A, Walter C (2001) Conifer genetic engineering: transgenic Pinus radiata (D. Don) and Picea abies (Karst) plants are resistant to the herbicide Buster. Plant Cell Rep 20:235–243
Charest PJ, Calero N, Lachance D, Dalta RSS, Duchesne LC, Tsang EWT (1993) Microprojectile-DNA delivery in conifer species: factors affecting assessment of transient gene expression using the β-glucuronidase reporter gene. Plant Cell Rep 12:189–193
Charest PJ, Devantier Y, Lachance D (1996) Stable genetic transformation of Picea mariana (Black spruce) via microprojectile bombardment. In Vitro Cell Dev Biol 32:91–99
Charity JA, Holland L, Grace LJ, Walter C (2005) Consistent and stable expression of the nptII, uidA and bar genes in transgenic Pinus radiata after Agrobacterium tumefaciens mediated transformation using nurse cultures. Plant Cell Rep (in press) (DOI: 10.1007/s00299-004-0851-6; published online 22 September 2004)
Christensen AH, Sharrock RA, Quail PH (1992) Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplast by electroporation. Plant Mol Biol 18:675–689
Clapham D, Demel P, Elfstrand M, Koop HU, Sabala I, Von Arnold S (2000) Gene transfer by particle bombardment to embryogenic cultures of Picea abies and the production of transgenic plantlets. Scand J For Res 15:151–160
Dunwell JM (2000) Transgenic approaches to crop improvement. J Exp Bot 51:487–496
Ellis DD, McCabe DE, McInnis S, Ramachandran R, Russell DR, Wallace KM, Martinell BJ, Roberts DR, Raffa KF, McCown BH (1993) Stable transformation of Picea glauca by particle acceleration. Biotechnology 11:84–89
Franck A, Guilley H, Jonard G, Richards K, Hirth L (1980) Nucleotide sequence of Cauliflower Mosaic Virus DNA. Cell 21:285
Gleave AP (1992) A versatile binary vector system with a T-DNA organizational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol Biol 20:1203–1207
Greenplate JT (1999) Quantification of Bacillus thuringiensis insect control protein CrylAc over time in Bollgard cotton fruit and terminals. J Econ Entomol 92:1377–1383
Harcourt RL, Kyozuka J, Floyd RB, Bateman KS, Tanaka H, Decroocq V, Llewellyn DJ, Zhu X, Peacock WJ, Dennis ES (2000) Insect- and herbicide-resistant transgenic eucalypts. Mol Breed 6:307–315
Hargreaves CL, Grace LJ, Holden DG (2002) Nurse culture for efficient recovery of cryopreserved Pinus radiata D. Don embryogenic cell lines. Plant Cell Rep 21:40–45
Hoefte H, Whiteley HR (1989) Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev 53:242–255
Hu JJ, Tian YC, Han YF, Li L, Zhang BE (2001) Field evaluation of insect-resistant Populus nigra trees. Euphytica 121:123–127
Huang Y, Diner AM, Karnosky DF (1991) Agrobacterium rhizogenes-mediated genetic transformation and regeneration of a conifer: Larix decidua. In Vitro Cell Dev Biol 27:201–207
James C (2002) Global status of commercialisation of transgenic crops: 2002. ISAAA briefs No.27. See http://www.isaaa.org
Jefferson RA, Burgess SM, Hirsh D (1986) beta-Glucuronidase from Escherichia coli as a gene-fusion marker. Proc Natl Acad Sci USA 83:8447–8451
Klimaszewska K, Devantier Y, Lachance D, Lelu M-A, Charest PJ (1997) Larix laricina (tamarack): somatic embryogenesis and genetic transformation. Can J For Res 27:538–550
Klimaszewska K, Lachance D, Pelletier G, Lelu M-A, Seguin A (2001) Regeneration of transgenic Picea glauca, P. mariana and P. abies after cocultivation of embryogenic tissue with Agrobacterium tumefaciens. In Vitro Cell Dev Biol Plant 37:748–755
Last DI, Brettell RIS, Chamberlain AM, Chaudhury AM, Larking PJ, Marsh EL, Peacock WJ, Dennis ES (1991) pEmu: an improved promoter for gene expression in cereal cells. Theor Appl Genet 81:581–588
Le VQ, Belles-Isles J, Dusabenyagasani M, Tremblay FM (2001) An improved procedure for production of white spruce (Picea glauca) transgenic plants using Agrobacterium tumefaciens. J Exp Bot 52:2089–2095
Levee V, Lelu M-A, Jouanin L, Cornu D, Pilate G (1997) Agrobacterium tumefaciens-mediated transformation of hybrid larch (Larix kaempferi × L. decidua) and transgenic plant regeneration. Plant Cell Rep 16:680–685
Levee V, Garin E, Klimaszewska K, Seguin A (1999) Stable genetic transformation of white pine (Pinus strobus L.) after cocultivation of embryogenic tissues with Agrobacterium tumefaciens. Mol Breed 5:429–440
Matsuki S, Kay M, Serin J, Floyd R, Scott JK (2001) Potential risk of accidental introduction of Asian gypsy moth (Lymantria dispar) to Australasia: effects of climatic conditions and suitability of native plants. Agric For Entomol 3:305–320
Nagel RJ, Manners JM, Birch RG (1992) Evaluation of an nptII assay for rapid detection and quantification of neomycin phosphotransferase II in transgenic plants. Plant Mol Biol Rep 10:263–272
Pena L, Seguin A (2001) Recent advances in the genetic transformation of trees. Trends Biotechnol 19:500–506
Sachs ES, Benedict JH, Stelly DM, Taylor JF, Altman DW, Berberich SA, Davis SK (1998) Expression and segregation of genes encoding CrylAc insecticidal proteins in cotton. Crop Sci 38:1–11
SAS Institute (1989) SAS/STAT users guide, version 6, 4th edn. SAS Institute, Cary, N.C.
Shelbourne CJA, Carson MJ, Wilcox MD (1989) New techniques in the genetic improvement of radiata pine. Commonw For Rev 68:3
Shin DI, Podila GK, Huang Y, Karnosky DF (1994) Transgenic larch expressing genes for herbicide and insect resistance. Can J For Res 24:2059–2067
Smith DR (1996) Growth medium. US patent no. 5, 565, 355
Su X-H, Zhang B-Y, Huang O-J, Huang L-J, Zhang X-H (2003) Advances in tree genetic engineering in China. In: Proc 12th World For Congr. Quebec City, Canada
Tang W, Newton RJ (2003) Genetic transformation of conifers and its application in forest biotechnology. Plant Cell Rep 22:1–15
Tang W, Samuels V (2002) Genetic transformation of Pinus taeda by particle bombardment. J For Res 13:91–97
Tang W, Tian Y (2003) Transgenic loblolly pine (Pinus taeda L.) plants expressing a modified δ-endotoxin gene of Bacillus thuringiensis with enhanced resistance to Dendrolimus punctatus Walker and Crypyothelea formosicola Staud. J Exp Bot 54:835–844
Trontin J-F, Harvengt L, Garin E, Lopez-Vernaza M, Arancio L, Hoebeke J, Canlet F, Pâques M (2002) Towards genetic engineering of maritime pine (Pinus pinaster Ait). Ann For Sci 59:687–697
Tzfira T, Zuker A, Altmann A (1998) Forest-tree biotechnology: genetic transformation and its application to future forests. Trends Biotechnol 16:439–446
Walter C (2002) Genetic engineering as a promising tool in forest biotechnology. In: Pandalai SG (ed) Recent research developments in plant biology, vol 2. Research Signpost, Kerala, India, pp 245–260
Walter C, Grace LJ (2000) Genetic engineering of conifers for plantation forestry: Pinus radiata transformation. In: Jain SM, Minocha SC (eds) Molecular biology of woody plants, vol 2. Kluwer, Dordrecht, pp 79–104
Walter C, Grace LJ, Wagner A, White DWR, Walden AR, Donaldson SS, Hinton H, Gardner RC, Smith DR (1998) Stable transformation and regeneration of transgenic plants of Pinus radiata D. Don. Plant Cell Rep 17:460–469
Walter C, Grace LJ, Donaldson SS, Moody J, Gemmell JE, van der Maas S, Kvaalen H, Lonneborg A (1999) An efficient biolistic transformation protocol for Picea abies embryogenic tissue and regeneration of transgenic plants. Can J For Res 29:1539–1546
Yanisch-Perron C, Vieira J, Messing J (1985) Improved M13 phage cloning vectors and host strains: nucleotide RT sequences of the M13mp18 and pUC19 vectors. RL Gene 33:103–119
Acknowledgements
The authors wish to thank Cathy Hargreaves, Cathie Reeves and Nancy Cranshaw for the initiation of embryogenic cell lines; Andrew McAlonan and Lorelle Phillips for assistance with the extraction of DNA from the plant tissue for Southern hybridisation; Karen Nielsen and Carmella Lee for assistance with the Southern hybridisation study; Judy Moody, Tomoko Pearson and Nancy Cranshaw for ELISA analysis; Susan van der Maas for maintenance of the plants in the containment glasshouse; Bill Faulds for providing neonate painted apple moth larvae; Charlie Low for statistical analysis; Tania Elder for typing this manuscript. This research was jointly funded by Fundacion Chile and the Foundation of Research Science and Technology
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S.A. Merkle
Rights and permissions
About this article
Cite this article
Grace, L.J., Charity, J.A., Gresham, B. et al. Insect-resistant transgenic Pinus radiata. Plant Cell Rep 24, 103–111 (2005). https://doi.org/10.1007/s00299-004-0912-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-004-0912-x