Abstract
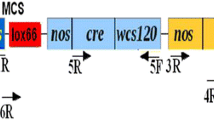
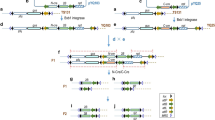
Removal of a selectable marker gene from genetically modified (GM) crops alleviates the risk of its release into the environment and hastens the public acceptance of GM crops. Here we report the production of marker-free transgenic rice by using a chemically regulated, Cre/loxP-mediated site-specific DNA recombination in a single transformation. Among 86 independent transgenic lines, ten were found to be marker-free in the T0 generation and an additional 17 lines segregated marker-free transgenic plants in the T1 generation. Molecular and genetic analyses indicated that the DNA recombination and excision in transgenic rice were precise and the marker-free recombinant T-DNA was stable and heritable.







Similar content being viewed by others
References
Coppoolse ER, Vroomen MJ, Roelofs D, Smit J, van Gennip F, Hersmus BJM, Nijkamp HJJ, van Kaaren MJJ (2003) Cre recombinase expression can result in phenotypic aberrations in plants. Plant Mol Biol 51:263–279
Cotsaftis O, Sallaud C, Breitler JC, Meynard D, Greco R, Pereira A, Guiderdoni E (2002) Transposon-mediated generation of T-DNA- and marker-free rice plants expressing a Bt endotoxin gene. Mol Breed 10:165–180
Dale EC, Ow DW (1991) Gene transfer with subsequent removal of the selection gene from the host genome. Proc Natl Acad Sci USA 88:10558–10562
Daley M, Knauf V, Summerfelt KR, Turner JC (1998) Co-transformation with one Agrobacterium tumefaciens strain containing two binary plasmids as a method for producing marker-free transgenic plants. Plant Cell Rep 17:489–496
Ebinuma H, Sugita K, Matsunaga E, Endo S, Yamada K, Komamine A (2001) Systems for the removal of a selection marker and their combination with a positive marker. Plant Cell Rep 20:383–392
Endo S, Sujita K, Sakai M, Tanaka H, Ebinuma H (2002) Single step transformation for generating marker-free transgenic rice using the ipt-type MAT vector system. Plant J 30:115–122
Gleave AP, Mitra DS, Mudge SR, Morris BAM (1999) Selectable marker-free transgenic plants without sexual crossing: transient expression of cre recombinase and use of a conditional lethal dominant gene. Plant Mol Biol 40:223–235
Goldsbrough AP, Lastrella CN, Yoder JI (1993) Transposition mediated re-positioning and subsequent elimination of marker genes from transgenic tomato. Biotechnology 11:1286–1292
Guo H-S, Fei J-F, Xie Q, Chua N-H (2003) A chemical-regulated inducible RNAi system in plants. Plant J 34:383–392
Hajdukiewicz PT (2001) Multiple pathways for Cre/lox-mediated recombination in plastids. Plant J 27:161–171
Hoa TTC, Bong BB, Huq E, Hodges TK (2002) Cre/lox site-specific recombination controls the excision of a transgene from the rice genome. Theor Appl Genet 104:518–525
Hobbs SLA, Warkentin TD, Delong CMO (1993) Transgene copy number can be positively or negatively associated with transgene expression. Plant Mol Biol 21:17–26
Hoff T, Schnorr KM, Mundy J (2001) A recombinase-mediated transcriptional induction system in transgenic plants. Plant Mol Biol 45:41–49
Kolesnik T, Szeverenyi I, Bachmann D, Kumar CS, Jiang S, Ramamoorthy R, Cai M, Ma ZG, Sundaresan V, Ramachandran S (2004) Establishing an efficient Ac/Ds tagging system in rice: large-scale analysis of Ds flanking sequences. Plant J 37:301–314
Komari T, Hiei Y, Saito Y, Murai N, Kumashiro T (1996) Vectors carrying two separate T-DNAs for co-transformation of higher plants mediated by Agrobacterium tumefaciens and segregation of transformants free from selection markers. Plant J 10:165–174
Lloyd AM, Davis RW (1994) Functional expression of the yeast FLP/FRT site-specific recombination system in Nicotiana tabacum. Mol Gen Genet 242:653–657
Lyznik LA, Mitchell JC, Hirayama L, Hodges TK (1993) Activity of yeast FLP recombinase in maize and rice protoplasts. Nucleic Acids Res 21:969–975
Maeser S, Kahmann R (1991) The Gin recombinase of phage Mu can catalyse site-specific recombination in plant protoplasts. Mol Gen Genet 230:170–176
McKnight T, Lillis M, Simpson R (1987) Segregation of genes transferred to one plant cell from two separate Agrobacterium strains. Plant Mol Biol 8:439–445
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Qin M, Bayley C, Stockton T, Ow DW (1994) Cre recombinase-mediated site-specific recombination between plant chromosomes. Proc Natl Acad Sci USA 91:1706–1710
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Sugita K, Kasahara T, Matsunaga E, Ebinuma H (2000) A transformation vector for the production of marker-free transgenic plants containing a single copy transgene at high frequency. Plant J 22:461–469
Yin Z, Wang GL (2000) Evidence of mutiple complex patterns of T-DNA integration into the rice genome. Theor Appl Genet 100:461–470
Zubko E, Scutt C, Meyer P (2000) Intrachromosomal recombination between attP regions as a tool to remove selectable marker genes from tobacco transgenes. Nat Biotechnol 18:442–445
Zuo J, Niu QW, Chua NH (2000) An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J 24:265–273
Zuo J, Niu QW, Moller SG, Chua NH (2001) Chemical-regulated, site-specific DNA excision in transgenic plants. Nat Biotechnol 19:157–161
Acknowledgements
The authors would like to thank N. -H. Chua (Rockefeller University, USA) for providing the pX7-GFP vector, S. Ramachandran for providing the pSSZ32 vector, D. Eriksson, and J.-H. Lee for technical assistance, N. Naqvi, Y. Hong and M. Griffith for critically reading the manuscript. Our research was supported by the Temasek Life Sciences Laboratory, Singapore, and the Agri-Food and Veterinary Authority of Singapore.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I.S. Chung
The first two authors contributed equally to the work
Rights and permissions
About this article
Cite this article
Sreekala, C., Wu, L., Gu, K. et al. Excision of a selectable marker in transgenic rice (Oryza sativa L.) using a chemically regulated Cre/loxP system. Plant Cell Rep 24, 86–94 (2005). https://doi.org/10.1007/s00299-004-0909-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-004-0909-5