Abstract
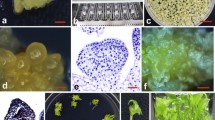
A population of transgenic plants was produced by the transformation of internodal explants of Solanum phureja, DB337/37 (the cultivar Mayan Gold) using an Agrobacterium tumefaciens LBA4404-based vector containing a phytoene synthase gene (crtB). The regeneration strategy utilised a two-step protocol, with a 12-day callus induction stage mediated by 1.07 μM α-napthaleneacetic acid (NAA), 7.10 μM zeatin riboside and 0.06 μM gibberellic acid (GA3), followed by a prolonged (up to 90 day) shoot induction stage on medium containing 0.11 μM NAA, 7.10 μM zeatin riboside and 0.06 μM GA3 supplemented with kanamycin at 50 mg l−1 as the selection agent. Southern analysis of the transgenic population revealed that the transgene copy number varied between one and five in the lines tested. Northern blot analysis showed significant expression of the introduced crtB gene in some lines during tuber development. Cytological analysis of the material showed a high incidence of chromosome doubling in the transgenic population with over 80% of all lines tested having doubled their chromosome complement during the transformation process.



Similar content being viewed by others
Abbreviations
- GA3:
-
Gibberellic acid
- MS:
-
Murashige and Skoog medium
- NAA:
-
α-Napthaleneacetic acid
References
van den Berg H, Faulks R, Granado HF, Hirschberg J, Olmedilla B, Sandmann G, Southon S, Stahl W (2000) The potential for the improvement of carotenoid levels in foods and the likely systemic effects. J Sci Food Agric 80:880–912
Bevan MW, Barker R, Goldsborough A, Jarvis M, Kavanagh T, Irurriaga G (1983). The structure and transcription start site of a major potato tuber protein gene. Nucleic Acid Res 14:4625–4638
Draper J, Scott R (1988) The isolation of plant nucleic acids. In: Draper J, Scott R, Armitage P, Walden R (eds) Plant genetic transformation and gene expression: a laboratory manual. Blackwell, Oxford, UK, pp 199–236
Ducreux L, Morris WL, Hedley PE, Shepherd T, Davies HV, Millam S, Taylor MA (2004) Metabolic engineering of high carotenoid potato tubers containing significant levels of β-carotene and lutein. J Exp Bot (in press)
Guerineau F, Woolston S, Brooks L, Mullineaux P (1988) An expression cassette for targeting foreign proteins into chloroplasts. Nucleic Acids Res 16:11380
Imai T, Aida R, Ishige T (1993). High-frequency of tetraploidy in Agrobacterium-mediated transformants regenerated from tuber disks of diploid potato lines. Plant Cell Rep 12:299–302
Lu WH, Haynes K, Wiley E, Clevidence B (2001). Carotenoid content and color in diploid potatoes. J Am Soc Hortic Sci 126:722–726
Millam S (2004) Agrobacterium-mediated transformation of potato. In: Curtis I (ed) Transgenic crops of the world. Kluwer, Dordrecht (in press)
Millam S, Davie P (2001) Somatic hybridization between Solanum tuberosum L. (potato) and Solanum phureja. In: Nagata T, Bajaj YPS (eds) Biotechnology in agriculture and forestry 49: somatic hybridization in crop improvement II. Springer, Berlin Heidelberg New York, pp 264–273
Morris WL, Ducreux L, Griffiths DW, Stewart D, Davies HV, Taylor MA (2004) Carotogenesis during tuber development and storage in potato. J Exp Bot 55:1–8
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Paz MM, Veilleux RE (1999). Influence of culture medium and in vitro conditions on shoot regeneration in Solanum phureja monoploids and fertility of regenerated doubled monoploids. Plant Breed 118:53–57
Romer S, Lubeck J, Kauder F, Steiger S, Adomat C, Sandmann G (2002). Genetic engineering of a zeaxanthin-rich potato by antisense inactivation and co-suppression of carotenoid epoxidation. Metab Eng 4:263–272
Snider KT, Veilleux RE (1984). Factors affecting variability in anther culture and in conversion of androgenic embryos of Solanum phureja. Plant Cell Tissue Org Cul 36:345–354
Trujillo C, Rodriguez-Arango E, Jaramillo S, Hoyos R, Orduz S, Arango R (2001). One-step transformation of two Andean potato cultivars (Solanum tuberosum L. subsp. Andigena). Plant Cell Rep 20:637–641
Acknowledgements
This work was funded by the Scottish Executive Environment and Rural Affairs Department. We wish to thank N. Misawa, Kirin Brewery, Kanagawa, Japan for the gift of the phytoene synthase gene (crtB) from Erwinia uredovara.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. Harwood
Rights and permissions
About this article
Cite this article
Ducreux, L.J.M., Morris, W.L., Taylor, M.A. et al. Agrobacterium-mediated transformation of Solanum phureja. Plant Cell Rep 24, 10–14 (2005). https://doi.org/10.1007/s00299-004-0902-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-004-0902-z