Abstract
Febuxostat is the drug used to treat hyperuricemia in patients with gout. Recently, the usage of Febuxostat has been controversial over the side effects in cardiovascular. The study aimed to comparatively analyze the risk of cardiovascular disease associated with febuxostat and allopurinol use in Korean patients with gout. A cohort study was conducted using national insurance claim data from the Health Insurance Review and Assessment Service (HIRA). Adult patients who were diagnosed with gout and prescribed febuxostat or allopurinol more than once from July 1, 2015, to June 30, 2018 were studied. The outcome was cardiovascular disease. Analysis was performed using Cox’s proportional hazard model following 1:1 propensity score matching to estimate the hazard ratio with a 95% confidence interval. In total, 90,590 patients were defined as the final study cohort who had an average follow-up of 467 days, including 28,732 and 61,858 patients in the febuxostat and allopurinol groups, respectively. After the 1:1 propensity score matching, the risk of cardiovascular disease in the febuxostat group was significantly higher than in the allopurinol group (HR: 1.17; 95% CI: 1.10–1.24). In the sensitivity analysis, the risk of cardiovascular disease in the febuxostat group was significantly higher than in the allopurinol group (HR: 1.09; 95% CI: 1.04–1.15). However, further sensitivity analysis showed no statistically significant difference between the febuxostat group and allopurinol group after adjusting for cardiovascular disease history before the index date. Similarly, no statistically significant difference was found between the two drugs in the subgroup analysis. Febuxostat was not associated with a significantly increased risk of cardiovascular disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gout is a metabolic disease in which the concentration of uric acid in the blood increases, leading to the deposition of uric acid crystals in tissues, such as joints and tendons, causing inflammation and pain. Its prevalence in Korea has increased steadily from 0.17% in 2001 to 0.4% in 2008 and 2.0% in 2015 according to the National Health Insurance Service [1]. Febuxostat is the primary drug used to treat hyperuricemia in patients with chronic gout. Febuxostat is an inhibitor of xanthine oxidase, like allopurinol, but its chemical structure differs from that of allopurinol, and hence, has no cross-reactions. Febuxostat lowers uric acid levels by selectively blocking xanthine oxidase [2]. Febuxostat was covered by national insurance as second-line therapy for cases where the efficacy of allopurinol was insufficient or there was a risk of hypersensitivity in Korea. However, since July 1, 2016, these insurance benefits were expanded to include febuxostat as first-line therapy [3].
The safety of febuxostat concerning cardiovascular disease has been a controversial issue. According to the results of phase 3 clinical trials such as FACT (2005) and APEX (2008) conducted before FDA approval, febuxostat had a higher rate of cardiovascular disease death and severe cardiovascular disease than allopurinol. However, there was no physiological mechanism to explain this, and the number of cases were small; so, it was necessary to confirm the relevance through additional clinical trials [4, 5]. In the subsequent F-153 (2008) clinical trial that added the proportion of patients with cardiovascular risk factors, it was found that febuxostat did not have more cardiovascular side effects than allopurinol. However, considering that the results were different from previous clinical trials, it received conditional approval (2009) with the risk of developing cardiovascular disease to be confirmed in a post-marketing assessment [6]. After approval by FDA, in the Cardiovascular Safety of Febuxostat and Allopurinol in Patients with Gout and Cardiovascular Morbidities (CARES) trial, the risks of cardiovascular-related and all-cause mortality were found to be 1.34 and 1.22 times higher with febuxostat than with allopurinol [7]. Thus, the US Food and Drug Administration (FDA) mandated that the gout drug febuxostat carries a boxed warning to alert clinicians and patients of the increased risk of cardiovascular death with the drug in February 2019 [8]. Furthermore, the American College of Rheumatology recommended allopurinol as the first-line therapy for gout treatment in the gout management guidelines in June 2020 [9]. On the other hand, the Ministry of Food and Drug Safety in Korea recommends the use of allopurinol for patients who do not have the HLA-B*5801 gene, as confirmed by genetic testing. Allopurinol has a risk of Severe Cutaneous Adverse Drug Reactions (SCAR) side effects when the HLA-B*5801 gene is present, and the retention rate of HLA-B*5801 gene in Koreans is 12.2%, which is higher than that of Westerners 1 ~ 2% [10].
Preclinical cardiovascular studies of febuxostat had shown no toxic effects related to cardiac rhythm and function [11,12,13,14,15], and to date, the mechanism underlying the risk of cardiovascular death is unclear [7]. Therefore, additional studies are required to determine causalities. In this study, febuxostat was compared with allopurinol in terms of association with cardiovascular disease in gout patients to provide evidence of drug safety in Korea.
Methods
Data source
This study used national insurance claim data from the Health Insurance Review and Assessment Service (HIRA) in Korea. The data includes about 98% of the entire nation’s population due to the universal coverage system in Korea (97% health insurance and 3% medical aid). HIRA data contain patient information related to healthcare services such as socio-demographics, diagnoses, treatments, procedures, pharmaceuticals, medical utilization and in-hospital deaths. More than 99% of the HIRA data were collected using an electronic data interchange system and contained about 120 information lists [16].
Study cohorts
A retrospective cohort study was performed using HIRA data from January 1, 2011, to June 30, 2018. Patients aged 19 years or older who were diagnosed with gout at least once and prescribed the study drugs (febuxostat or allopurinol) more than once from July 1, 2015, to June 30, 2018 (3 years) were selected. We identified the index date by the first prescription date of febuxostat or allopurinol for each patient and classified them into two groups (febuxostat exposed group or allopurinol control group). We excluded patients who were prescribed the study drugs (febuxostat or allopurinol) from January 1, 2011, to June 30, 2015. We also excluded patients younger than 19 years and those with a history of end-stage kidney disease/dialysis, myeloproliferative disease, xanthinuria, peptic ulcer, cancer, rheumatoid arthritis, hepatitis B, hepatitis C, liver cirrhosis, and HIV positivity before the index date (Fig. 1).
Exposure and outcomes
We included patients who were prescribed the drugs of interest more than once after gout diagnosis. Since July 1, 2016, the insurance benefits for febuxostat were expanded in Korea; thus, we re-classified patients who were prescribed both drugs. We excluded patients who were cross-prescribed both drugs more than twice. Those who were cross-prescribed both drugs once were included in the latest drug use group if the second drug was prescribed less than 365 days after the first drug. We excluded patients who were prescribed the second drug more than 365 days after the first drug because various other confounding factors could have affected the cross-prescription of the study drugs. For this definition of exposure, we referred to a previous study (Fig. 2) [17].
The primary outcome of the study was defined as the first diagnosis of a cardiovascular disease, such as myocardial infarction, ischemic heart disease, stroke, transient ischemic attack, heart failure, coronary revascularization, and all-cause death observed during the follow-up period. Secondary outcomes were individual components of the primary outcome. Additionally, when the outcomes were observed multiple times in one patient, only the first case was included in the analysis (Appendix Table 9).
Covariates
The confounding variables were as follows: sex, age, medication history for 1 year before the index date (such as angiotensin-converting enzyme inhibitors, calcium channel blockers, angiotensin receptor blockers, diuretics, hypoglycemic agents, anti-hyperlipidemic drugs, systemic steroids, probenecid, colchicine, and nonsteroidal anti-inflammatory drugs [NSAIDs]), disease history (such as renal failure, kidney stones, angina, diabetes, peripheral artery disease, degenerative arthritis, hypertension, hyperlipidemia, obesity, smoking, and Charlson comorbidity index [CCI]), and cardiovascular history before the index date (myocardial infarction, ischemic heart disease, stroke, transient ischemic attack, heart failure, and coronary revascularization).
Statistical analysis
All statistical analyses were performed using the SAS Enterprise Guide 6.1 software (SAS Institute Inc., Cary, NC, United States), and significance was set at P < 0.05. To compare the differences in baseline characteristics between the febuxostat and allopurinol users, we examined the frequencies and percentages of the categorical variables and compared them using the chi-square test, and in some cases, Fisher’s exact test. Next, we calculated the means and standard deviations (SD) for the continuous variables. Then, we used propensity score (PS) matching (caliper 1:1 matching) to reduce the potential selection bias in observational studies and balance the distribution between the two groups by excluding confounding variables [18]. We used the Cox proportional hazard regression and estimated the hazard ratio (HR) with a 95% confidence interval (CI) for the risk of cardiovascular disease associated with febuxostat and allopurinol. As a sensitivity analysis, we limited the study period (from July 1, 2016, to June 30, 2018) to consider the time when febuxostat insurance benefit was expanded as the first-line therapy for gout in Korea and analyzed the risk of cardiovascular disease with febuxostat group compared to that with allopurinol group. We also compared the risk of cardiovascular disease between the two drug groups according to cardiovascular disease history, using the same method of PS matching (Appendix Tables 4, 5, 6, 7).
Results
Patient characteristics
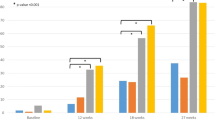
In total, 90,590 patients were defined in the final study cohort, including 28,732 (31.7%) and 61,858 (68.3%) patients in the febuxostat and allopurinol groups, respectively. This baseline cohort comprised 90.7% males, and the mean age of the febuxostat group was 46.6 years, which was higher than that of the allopurinol group (46.3 years). Patients in the febuxostat group had a higher prevalence of renal failure, diabetes, hypertension, and hyperlipidemia compared to the allopurinol group. After 1:1 PS matching, 27,761 patients were grouped in the febuxostat group and 27,761 in the allopurinol group. All baseline covariates (medication history, CCI, and disease history) including the cardiovascular risk factors were well balanced with a standardized difference in the prevalence of < 0.1 after PS matching (Table 1).
Risk of cardiovascular disease associated with febuxostat and allopurinol groups
After PS matching, the risk of cardiovascular disease in the febuxostat group was significantly higher than that in the allopurinol group (HR: 1.17; 95% CI: 1.10–1.24). In the secondary outcome analysis, the risks of ischemic heart disease, stroke, and heart failure in the febuxostat group were significantly higher than those in the allopurinol group; ischemic heart disease: HR 1.19, 95% CI 1.05–1.35; stroke: HR 1.12, 95% CI 1.01–1.25, and heart failure: HR 1.16, 95% CI 1.06–1.27, respectively (Table 2).
Sensitivity analysis
In total, 68,317 patients (29,412 and 38,905 patients in the febuxostat and allopurinol groups, respectively) received febuxostat and allopurinol between 2016 and 2018. After 1:1 PS matching, 26,962 patients in the febuxostat group and 26,962 in the allopurinol group were identified. After PS matching, the risk of cardiovascular disease in the febuxostat group was significantly higher than that in the allopurinol group (HR: 1.09; 95% CI: 1.04–1.15). However, after adjusting for cardiovascular disease history before the index date, the risk of cardiovascular disease was not different between the two groups (HR: 1.04; 95% CI: 0.99–1.10) (Table 3).
Subgroup analysis
In the subgroup analysis considering those with and without a cardiovascular disease history before the index date, there was no statistically significant difference in the risk of cardiovascular disease between the febuxostat and allopurinol groups (Appendix Tables 4, 5, 6, 7). For the primary outcome, cardiovascular disease, the HR for the febuxostat group compared to the allopurinol group were 1.02 (95% CI: 0.95–1.10) in those with a cardiovascular disease history and 1.11 (95% CI: 0.99–1.24) in those without a cardiovascular disease history. The sensitivity analysis results were also similar between the two groups.
Discussion
Based on our results, the risk of cardiovascular disease in the febuxostat group was significantly higher than that in the allopurinol group. However, in the sensitivity analysis, after adjusting for cardiovascular disease history before the index date, there was no statistically significant difference in the risk of cardiovascular disease between the febuxostat and allopurinol groups. Besides, in the subgroup analysis including those with and without a cardiovascular disease history, there was no statistically significant difference. From the combined results of the analyses, we concluded that febuxostat was not associated with a higher risk of cardiovascular disease than allopurinol. In addition, we have also performed subgroup analysis by sex and age. The subgroup analysis result showed a trend similar to the main analysis result. In our study, the rates of medication history, CCI, disease history, and cardiovascular disease history were significantly higher in the febuxostat group than in the allopurinol group from 2015 to 2018. In contrast, the differences in the rates between the two groups decreased after the expansion of the insurance benefits to febuxostat used as the first-line therapy. The difference in patient characteristics and drug use patterns according to the expansion of the insurance benefits may have affected the results (Appendix Table 8). Moreover, no statistically significant difference was observed between the two groups in the secondary outcomes after PS matching and adjusted for the cardiovascular disease history. Thus, there was no association between the incidence of cardiovascular disease and febuxostat.
According to a study by Zhang et al. [17], there was no statistically significant difference in the association of hospitalization for myocardial infarction or stroke between the febuxostat and allopurinol groups. Besides, the subgroup analysis stratifying those with and without baseline cardiovascular disease (including myocardial infarction, stroke, coronary revascularization, and all-cause death) showed no statistically significant difference between the febuxostat and allopurinol groups. This finding was consistent with that of our study. Moreover, in a retrospective cohort study by Kang et al. [19], the risk of cardiovascular disease (including myocardial infarction, stroke, transient ischemic attack, and coronary revascularization) was not different between the two groups. Kang et al. [19] used claim data from 2002 to 2015 in Korea, and only analyzed the period in which febuxostat was covered by insurance as a second-line therapy. However, our study used recent claim data (July 1, 2015, to June 30, 2018) including data from the period in which the insurance coverage benefit was expanded to febuxostat as first-line therapy in Korea (July 1, 2016); we analyzed the data using sensitivity analysis. In other words, this study observed the effects of febuxostat and allopurinol on the risk of cardiovascular disease under a condition in which both drugs were equally considered as first-line therapy in clinical settings. A recent meta-analysis study found that febuxostat use was not associated with an increased risk of cardiovascular events and death, all-cause mortalities [20, 21]. In addition, febuxostat was found not to be associated with an increased risk of death or serious adverse events compared to allopurinol in a recent FAST (2020) study [22]. Nevertheless, in the CARES trial [7], the risks of cardiovascular-related and all-cause mortality were 1.34 and 1.22 times higher with febuxostat than with allopurinol. The CARES trial is a randomly assigned clinical trial, which was conducted using a study method that minimized selection bias by ensuring that the study cohort had an equal probability of being assigned to each group and by normalizing all factors other than the drugs administered. However, 56.6% of patients discontinued the trial regimen prematurely, and 45.0% of patients discontinued follow-up, making it difficult to judge if the selection bias was minimized [23]. Moreover, in the CARES trial, the risk of cardiovascular-related mortality was different when a subgroup analysis was performed including the combination of the study drugs with low-dose aspirin or NSAIDs. In this study, PS matching was performed including aspirin and NSAIDs, as well as diseases and medication use that may affect cardiovascular diseases and hyperuricemia risk, thereby, minimizing potential selection bias. However, the rates of medication history (anti-hyperlipidemic agents, hypoglycemic agents, and hypotensors) and history of diseases such as kidney failure, angina, diabetes, hypertension, hyperlipidemia, and cardiovascular disease, especially heart failure, were higher in the febuxostat group than in the allopurinol group. These characteristics may have affected the risk for cardiovascular disease [24]. Furthermore, the incidences of ischemic heart disease and heart failure were higher in the febuxostat group than in the allopurinol group. Therefore, febuxostat use in patients with related conditions would need to be carefully monitored.
The key strength of this study is that it represents a large population using national claims data. The HIRA data includes about 98% of the entire nation’s population due to the universal coverage system of Korea (97% health insurance and 3% medical aid). Moreover, we included all patients aged 19 years and above in the study cohort, and the results of this study are applicable to all adult patients with gout. In particular, the results of this study are meaningful in that they differ from previous studies that analyzed the risk of cardiovascular disease associated with febuxostat in specific age groups. We also sought to minimize the effect of confounding factors, such as underlying diseases and medication use, which are known to be related to cardiovascular disease and hyperuricemia risk, by applying statistical methods such as PS matching.
Despite these strengths, this study has some limitations. First, as is inherent to any observational study, socioeconomic and clinical confounding factors that were unmeasured or unpredicted may have affected the results. Therefore, clinical characteristics such as cardiovascular complications, disease severity, and serum uric acid levels should be considered comprehensively when using febuxostat in patients with gout. Second, we obtained the maximum study period considering the approval date of febuxostat (June 2009) and the date of expansion of insurance coverage to febuxostat as first-line therapy (July 1, 2016). However, it was insufficient to assess the association between cardiovascular disease and long-term febuxostat use. Therefore, the long-term safety of febuxostat and follow-up studies should be continuously monitored. Third, we compared the risk of cardiovascular disease associated with febuxostat to that related to allopurinol and therefore, cannot conclude on the absolute impact of febuxostat use on the risk of cardiovascular disease. Therefore, it would be unreasonable to consider the risk of cardiovascular disease as a decisive factor in choosing between febuxostat and allopurinol. Finally, the association between febuxostat and cardiovascular death, which has been a controversial safety issue in other countries, could not be defined because of the nature of the insurance claim data.
In conclusion, there was a significant difference in the risk of cardiovascular disease between gout patients who were treated with febuxostat and those with allopurinol. However, after adjusting for the history of cardiovascular disease, and considering the period during which the insurance benefits of febuxostat were expanded to its use as a first-line therapy (July 1, 2016), a causal link between febuxostat and febuxostat-associated cardiovascular disease events was not established. However, the FDA has restricted the use of febuxostat to those patients who experience side effects and show no treatment effects of allopurinol, based on the results of the CARES trial, and the American College of Rheumatology has recommended allopurinol as the first-line therapy for gout treatment in the gout management guidelines in June 2020. In addition, in Korea, instructions specify that febuxostat should be carefully administered in patients with ischemic heart disease or congestive heart failure. Therefore, long-term follow-up is required when febuxostat is prescribed for patients with cardiovascular disease.
References
JS P. Assessment of prevalence, incidence and risk factors associated with metabolic syndrome of gout using the National Health Insurance claim data: National Health Insurance Services Ilsan Hospital; 2017.
Js S (2018) New classification criteria and guideline for management of gout. Korean J Med 93(4):344–350
MFDS Integrated pharmaceutical information system [https://nedrug.mfds.go.kr]. Ministry of Food and Drug Safety. [cited February 25, 2019]
Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA et al (2005) Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 353(23):2450–2461 (PubMed PMID: 16339094)
Schumacher HR Jr, Becker MA, Wortmann RL, Macdonald PA, Hunt B, Streit J et al (2008) Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum 59(11):1540–1548 (PubMed PMID: 18975369)
FDA. Center for Drug Evaluation and Research Application number: NDA 21–856, Cross Discipline Team Leader(CDTL) Review 2009 [updated Accessed November 1, 2021]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/021856s000_CrossR.pdf.
White WB, Saag KG, Becker MA, Borer JS, Gorelick PB, Whelton A et al (2018) Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med 378(13):1200–1210 (PubMed PMID: 29527974)
FDA adds boxed warning for increased risk of death with gout medicine Uloric (febuxostat): US Food and Drug Administration; 2019 [updated February 21, 2019; cited 2021 July 12 ]. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-increased-risk-death-gout-medicine-uloric-febuxostat.
FitzGerald JD, Dalbeth N, Mikuls T, Brignardello-Petersen R, Guyatt G, Abeles AM et al (2020) 2020 American college of rheumatology guideline for the management of gout. Arthritis Care Res 72(6):744–760 (PubMed PMID: 32391934)
Integrated pharmaceutical information system: Ministry of Food and Drug Safety; 2017 [cited 2021 November 1]. Available from: https://nedrug.mfds.go.kr.
Grewal HK, Martinez JR, Espinoza LR (2014) Febuxostat: drug review and update. Expert Opin Drug Metab Toxicol 10(5):747–758 (PubMed PMID: 24684266)
Sanchez-Lozada LG, Tapia E, Bautista-Garcia P, Soto V, Avila-Casado C, Vega-Campos IP et al (2008) Effects of febuxostat on metabolic and renal alterations in rats with fructose-induced metabolic syndrome. Am J Physiol Renal Physiol 294(4):F710–F718 (PubMed PMID: 18216151)
Zhao L, Roche BM, Wessale JL, Kijtawornrat A, Lolly JL, Shemanski D et al (2008) Chronic xanthine oxidase inhibition following myocardial infarction in rabbits: effects of early versus delayed treatment. Life Sci 82(9–10):495–502 (PubMed PMID: 18215719)
Xu X, Hu X, Lu Z, Zhang P, Zhao L, Wessale JL et al (2008) Xanthine oxidase inhibition with febuxostat attenuates systolic overload-induced left ventricular hypertrophy and dysfunction in mice. J cardiac failure. 14(9):746–753
Hou M, Hu Q, Chen Y, Zhao L, Zhang J, Bache RJ (2006) Acute effects of febuxostat, a nonpurine selective inhibitor of xanthine oxidase, in pacing induced heart failure. J Cardiovasc Pharmacol 48(5):255–263 (PubMed PMID: 17110808)
Kim JA, Yoon S, Kim LY, Kim DS (2017) Towards actualizing the value potential of korea health insurance review and assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean med sci 32(5):718–728
Zhang M, Solomon DH, Desai RJ, Kang EH, Liu J, Neogi T et al (2018) Assessment of cardiovascular risk in older patients with gout initiating febuxostat versus allopurinol: population-based cohort study. Circulation 138(11):1116–1126
Rosenbaum PR, Rubin DB (1983) The central role of the propensity score in observational studies for causal effects. Biometrika 70(1):41–55
Kang EH, Choi HK, Shin A, Lee YJ, Lee EB, Song YW et al (2019) Comparative cardiovascular risk of allopurinol versus febuxostat in patients with gout: a nation-wide cohort study. Rheumatology 58(12):2122–2129 (PubMed PMID: 31098635)
Deng H, Zhang BL, Tong JD, Yang XH, Jin HM (2021) Febuxostat use and risks of cardiovascular disease events, cardiac death, and all-cause mortality: metaanalysis of randomized controlled trials. J Rheumatol 48(7):1082–1089 (PubMed PMID: 32801136)
Gao L, Wang B, Pan Y, Lu Y, Cheng R (2021) Cardiovascular safety of febuxostat compared to allopurinol for the treatment of gout: A systematic and meta-analysis. Clin cardiol 44(7):907–916
Mackenzie IS, Ford I, Nuki G, Hallas J, Hawkey CJ, Webster J et al (2020) Long-term cardiovascular safety of febuxostat compared with allopurinol in patients with gout (FAST): a multicentre, prospective, randomised, open-label, non-inferiority trial. Lancet 396(10264):1745–1757 (PubMed PMID: 33181081)
Jansen TLTA, Janssen M (2019) Gout lessons from 2018: CARES, a direct comparison of febuxostat vs allopurinol, and CANTOS, IL1 blocker for cardiovascular risk minimisation. Clin Rheumatol 38(1):263–265
Su CY, Shen LJ, Hsieh SC, Lin LY, Lin FJ (2019) Comparing Cardiovascular Safety of Febuxostat and Allopurinol in the Real World: A Population-Based Cohort Study. Mayo Clin Proc 94(7):1147–1157 (PubMed PMID: 31272565)
Acknowledgements
We would like to thank the Big Data Department of the Health Insurance Review and Assessment Service (HIRA) for its support.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
HJ and EC are co-first authors and contributed equally. HJ and EC contributed to the conceptualization, methodology, software, statistical analysis, and writing of the original draft. MY contributed to supervision. BK contributed to the supervision, project administration, writing, reviewing, and editing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study protocol was approved by the institutional review board (IRB) of the Korea Institute of Drug Safety and Risk Management (KIDS; KIDS-2019–4). Informed consent was waived by the IRB.
Consent to participate
Since only anonymized data were used, informed consent by the insured individuals was not required.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
See Tables 4, 5, 6, 7, 8, 9, 10.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jeong, H., Choi, E., Suh, A. et al. `Risk of cardiovascular disease associated with febuxostat versus allopurinol use in patients with gout: a retrospective cohort study in Korea. Rheumatol Int 43, 265–281 (2023). https://doi.org/10.1007/s00296-022-05222-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-022-05222-0