Abstract
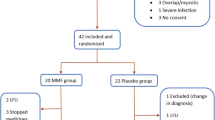
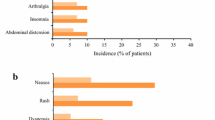
To assess the efficacy and safety of pirfenidone in systemic sclerosis-related interstitial lung disease (SSc-ILD). This was a double-blind, randomised, placebo-controlled, pilot study. Subjects with SSc-ILD and forced vital capacity (FVC) between 50 and 80% of the predicted (%pred) value were randomised in 1:1 ratio to receive either pirfenidone (2400 mg/day) or placebo for 6 months. Primary outcome was the proportion of subjects with either stabilisation or improvement in FVC at 6 months. Secondary outcomes were the absolute change in the %pred FVC, Mahler’s dyspnoea index, 6-min walk distance (6MWD), modified Rodnan skin score (MRSS) and serum levels of tumour necrosis factor α (TNF-α) and transforming growth factor β (TGF-β). Thirty-four subjects with median (range) age of 41 (20–63) years (91.2% women) and median (range) %pred FVC of 65 (51–78) were enrolled. Stabilisation/improvement in FVC was seen in 16 (94.1%) and 13 (76.5%) subjects in the pirfenidone and placebo groups, respectively (p = 0.33). The median (range) absolute change in %pred FVC was − 0.55 (− 9 to 7%) and 1.0 (− 42 to 11.5%) in the treatment and control groups, respectively (p = 0.51). The changes in 6MWD, dyspnoea scores, MRSS, and levels of TNF-α and TGF-β were not significantly different between groups. Common adverse events were gastrointestinal disturbances and skin rash. We failed to find a significant beneficial effect of pirfenidone over placebo in improving/stabilising FVC, exercise capacity, symptoms, or skin disease. Study is underpowered to provide conclusive evidence. Larger studies with longer follow-up periods are required.


Similar content being viewed by others
References
Steen VD, Medsger TA (2007) Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 66(7):940–944
Giacomelli R, Valentini G, Salsano F, Cipriani P, Sambo P, Conforti ML et al (2002) Cyclophosphamide pulse regimen in the treatment of alveolitis in systemic sclerosis. J Rheumatol 29(4):731–736
Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE et al (2006) Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med 354(25):2655–2666
Tashkin DP, Roth MD, Clements PJ, Furst DE, Khanna D, Kleerup EC et al (2016) Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med 4(9):708–719
Oku H, Shimizu T, Kawabata T, Nagira M, Hikita I, Ueyama A et al (2008) Antifibrotic action of pirfenidone and prednisolone: Different effects on pulmonary cytokines and growth factors in bleomycin-induced murine pulmonary fibrosis. Eur J Pharmacol 590(1–3):400–408
Nakazato H, Oku H, Yamane S, Tsuruta Y, Suzuki R (2002) A novel anti-fibrotic agent pirfenidone suppresses tumor necrosis factor-α at the translational level. Eur J Pharmacol 446(1):177–185
Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D et al (2011) Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 377(9779):1760–1769
King TE Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK et al (2014) A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 370(22):2083–2092
Herzog EL, Mathur A, Tager AM, Feghali-Bostwick C, Schneider F, Varga J (2014) Interstitial lung disease associated with systemic sclerosis and idiopathic pulmonary fibrosis. Arthritis Rheumatol Hoboken NJ 66(8):1967–1978
Mukerjee D, George DS, Coleiro B, Knight C, Denton CP, Davar J et al (2003) Prevalence and outcome in systemic sclerosis associated pulmonary arterial hypertension: application of a registry approach. Ann Rheum Dis 62(11):1088–1093
Khanna D, Furst DE, Clements PJ, Allanore Y, Baron M, Czirjak L et al (2017) Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J Scleroderma Relat Disord 2(1):11–18
Mahler DA, Weinberg DH, Wells CK, Feinstein AR (1984) The measurement of dyspnea: contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest 85(6):751–758
Burt RK, Shah SJ, Dill K, Grant T, Gheorghiade M, Schroeder J et al (2011) Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): an open-label, randomised phase 2 trial. Lancet 378(9790):498–506
Sullivan KM, Goldmuntz EA, Keyes-Elstein L, McSweeney PA, Pinckney A, Welch B et al (2018) Myeloablative autologous stem-cell transplantation for severe scleroderma. N Engl J Med 378(1):35–47
Udwadia ZF, Mullerpattan JB, Balakrishnan C, Richeldi L (2015) Improved pulmonary function following pirfenidone treatment in a patient with progressive interstitial lung disease associated with systemic sclerosis. Lung India Off Organ Indian Chest Soc 32(1):50–52
Miura Y, Tsunoda Y, Tanaka T, Takoi H, Yatagai Y, Rin S et al (2012) The efficacy of pirfenidone in scleroderma related interstitial lung disease. Eur Respir J 40:P3652
Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K et al (2005) Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 171(9):1040–1047
Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, et al (2019) Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med
Khanna D, Albera C, Fischer A, Khalidi N, Raghu G, Chung L, et al (2016) An open-label, phase II study of the safety and tolerability of pirfenidone in patients with scleroderma-associated interstitial lung disease: the LOTUSS Trial. J Rheumatol. jrheum.151322.
Costabel U, Albera C, Cohen A, Bradford W, King T, Noble P et al (2011) The long-term safety of pirfenidone in patients with idiopathic pulmonary fibrosis (IPF): interim data from the RECAP extension study. Eur Respir J 38(Suppl 55):174
Salih GN, Shaker SB, Madsen HD, Bendstrup E (2016) Pirfenidone treatment in idiopathic pulmonary fibrosis: nationwide Danish results. Eur Clin Respir J 3(1):32608
Harari S, Caminati A, Albera C, Vancheri C, Poletti V, Pesci A et al (2015) Efficacy of pirfenidone for idiopathic pulmonary fibrosis: an Italian real life study. Respir Med 109(7):904–913
Bando M, Yamauchi H, Ogura T, Taniguchi H, Watanabe K, Azuma A et al (2016) Clinical experience of the long-term use of pirfenidone for idiopathic pulmonary fibrosis. Intern Med 55(5):443–448
Tzouvelekis A, Karampitsakos T, Ntolios P, Tzilas V, Bouros E, Markozannes E et al (2017) Longitudinal “Real-World” outcomes of pirfenidone in idiopathic pulmonary fibrosis in Greece. Front Med 4:213
Uehara M, Enomoto N, Oyama Y, Suzuki Y, Kono M, Furuhashi K et al (2018) Body size-adjusted dose analysis of pirfenidone in patients with interstitial pneumonia. Respirology 23(3):318–324
Hostettler K, Zhong J, Tamm M, Lardinois D, Roth M (2014) Effect of pirfenidone on TGF-β-induced pro-fibrotic effects in primary human lung cells derived from patients with idiopathic pulmonary fibrosis. Eur Respir J 44(Suppl 58):P763
Distler O, Highland KB, Gahlemann M, Azuma A, Fischer A, Mayes MD et al (2019) Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med 380(26):2518–2528
Denton CP, Hughes M, Gak N, Vila J, Buch MH, Chakravarty K et al (2016) BSR and BHPR guideline for the treatment of systemic sclerosis. Rheumatol Oxf Engl 55(10):1906–1910
Kowal-Bielecka O, Fransen J, Avouac J, Becker M, Kulak A, Allanore Y et al (2017) Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis 76(8):1327–1339
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Acharya, N., Sharma, S.K., Mishra, D. et al. Efficacy and safety of pirfenidone in systemic sclerosis-related interstitial lung disease—a randomised controlled trial. Rheumatol Int 40, 703–710 (2020). https://doi.org/10.1007/s00296-020-04565-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-020-04565-w