Abstract
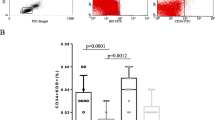
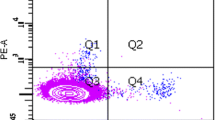
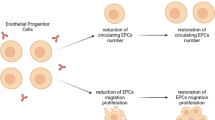
Circulating endothelial progenitor cells (CEPCs) play an important role in the process of atherosclerosis. Most previous studies on CEPC in systemic lupus erythematosus (SLE) patients were on their number and some functions and the results were not consistent. No studies on their anti-inflammatory function and integrated status were reported. The purpose of this study was to determine the number, function (including anti-inflammatory function), and the integrated status of CEPCs in active SLE patients. The study was performed in 35 active SLE patients (28 females, 7 males) and 35 age-and gender-matched healthy controls. CEPC number was determined by Fluorescence-Activated Cell Sorting. Proliferation capacity of CEPC was assessed by PCNA staining. Adhesion capacity of CEPC to fibronectin and adhesion capacity of THP1 cell to CEPC were determined by cell adhesion assay. Migratory capacity of CEPC was measured by transwell chamber assay and the potential to form tubes on Matrigel of CEPC was determined by in vivo tube formation on Matrigel test. The expression of inducible nitric oxide synthase (iNOS) and interleukin-6 (IL-6) assessed by quantitative PCR as well as the expression of intercellular adhesion molecule-1 (ICAM-1) and phosphorylated-Akt (p-Akt) assessed by western-blotting were used to evaluate the anti-inflammatory function and cell status of CEPCs. The number of CEPC in SLE patients was not different from that in control (p > 0.05). Proliferation capacity of CEPC was decreased in active SLE patients (p = 0.027). Adhesion capacity of CEPC to fibronectin was decreased (p = 0.04) in SLE patients and adhesion capacity of THP1 cell to CEPC was increased in SLE patients (p < 0.001). Migratory activity was reduced in patient CEPCs (p < 0.001). Capacity of CEPCs to form tube on Matrigel was decreased in SLE patients (p < 0.001). Expression of iNOS and IL-6 (p < 0.001, p = 0.006, respectively) and ICAM-1 were increased in CEPC of SLE patients and expression of p-Akt was decreased in CEPC of SLE patients. Our data show that CEPC number in active SLE patients was not significantly different from healthy controls, but their functions were partly impaired, including proliferation, adhesion, migration, and tube formation. Bad cell status and increased susceptibility to inflammatory process of CEPCs in active SLE were also observed in our study.









Similar content being viewed by others
References
Bessant R, Hingorani A, Patel L, MacGregor A, Isenberg DA, Rahman A (2004) Risk of coronary heart disease and stroke in a large British cohort of patients with systemic lupus erythematosus. Rheumatology 43:924–929
Esdaile JM, Abrahamowicz M, Grodzicky T et al (2001) Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum 44:2331–2337
Hristov M, Weber C (2009) Progenitor cell trafficking in the vascular wall. J Thromb Haemost 7(Suppl 1):31–34
Hristov M, Zernecke A, Schober A et al (2008) Adult progenitor cells in vascular remodeling during atherosclerosis. Biol Chem 389(7):837–844
Moreno PR, Sanz J, Fuster V (2009) Promoting mechanisms of vascular health: circulating progenitor cells, angiogenesis, and reverse cholesterol transport. J Am Coll Cardiol 53(25):2315–2323
Grisar J, Steiner CW, Bonelli M et al (2008) Systemic lupus erythematosus patients exhibit functional deficiencies of endothelial progenitor cells. Rheumatology 47:1476–1483
Westerweel PE, Luijten RK, Hoefer et al (2007) Haematopoietic and endothelial progenitor cells are deficient in quiescent systemic lupus erythematosus. Ann Rheum Dis 66:865–870
Lee PY, Li Y, Richards HB et al (2007) Type I interferon as a novel risk factor for endothelial progenitor cell depletion and endothelial dysfunction in systemic lupus erythematosus. Arthritis Rheum 56:3759–3769
Robak E, Kierstan M, Cebula B et al (2009) Circulating endothelial cells and angiogenic proteins in patients with systemic lupus erythematosus. Lupus 18:332–341
Hochberg MC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus [letter]. Arthritis Rheum 40:1725
Urbich C, Dimmeler S (2005) Risk factors for coronary artery disease, circulating endothelial progenitor cells, and the role of HMG-CoA reductase inhibitors. Kidney Int 67:1672–1676
Tepper OM, Galiano RD, Capla JM et al (2002) Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 26:2781–2786
Wang N, Verna L, Liao H, Ballard A, Zhu Y, Stemerman MB (2001) Adenovirus-mediated overexpression of dominant-negative mutant of c-Jun prevents intercellular adhesion molecule-1 induction by LDL: a critical role for activator protein-1 in endothelial activation. Arterioscler Thromb Vasc Biol 21:1414–1420
Bruce IN (2005) Atherogenesis and autoimmune disease: the model of lupus. Lupus 14:687–690
Murakami J, Li TS, Ueda K, Tanaka T (2009) Inhibition of accelerated tumor growth by blocking the recruitment of mobilized endothelial progenitor cells after chemotherapy. Int J Cancer 124(7):1685–1692
Wang CH, Cherng WJ, Yang NI et al (2008) Cyclosporine increases ischemia-induced endothelial progenitor cell mobilization through manipulation of the CD26 system. Am J Physiol Regul Integr Comp Physiol 294(3):R811–R818
Grisar J, Aletaha D, Steiner CW et al (2007) Endothelial progenitor cells in active rheumatoid arthritis: effects of tumour necrosis factor and glucocorticoid therapy. Ann Rheum Dis 66(10):1284–1288
Potvin F, Petitclerc E, Marcau F, Poubelle PE (1997) Mechanisms of action of antimalarials in inflammation: induction of apoptosis in human endothelial cells. J Immunol 158:1872–1879
Asahara T, Murohara T, Sullivan A et al (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275:964–967
Hingorani AD, Cross J, Kharbanda RK et al (2000) Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation 102:994–999
Ross R (1999) Atherosclerosis: an inflammatory disease. N Engl J Med 340:115–126
Szekanecz Z, Koch AE (2007) Mechanism of disease: angiogenesis in inflammatory diseases. Nat Clin Pract Rheumatol 3:635–643
Shiojima I, Walsh K (2002) Role of Akt Signaling in vascular homeostasis and angiogenesis. Circ Res 90:1243–1250
Kleinert H, Pautz A, Linker K, Schwarz PM (2004) Regulation of the expression of inducible nitric oxide synthase. Eur J Pharmacol 500:255–266
Acknowledgments
This paper is supported by the following funds: (1) Seed Foundation of Peking University Third Hospital; (2) Key Program Foundation of Peking University Third Hospital; (3) National Natural Science Foundation of China (30600209). Department of Rheumatology and Immunology, Peking University Third Hospital is the department to which the work should be attributed.
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Both Xiao Li Deng and Xiao Xia Li are the first authors of this paper.
Rights and permissions
About this article
Cite this article
Deng, X.L., Li, X.X., Liu, X.Y. et al. Comparative study on circulating endothelial progenitor cells in systemic lupus erythematosus patients at active stage. Rheumatol Int 30, 1429–1436 (2010). https://doi.org/10.1007/s00296-009-1156-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-009-1156-4