Abstract
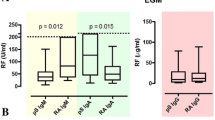
Human T cell lymphotropic virus type 1 (HTLV-1) is endemic in many regions of the world, including Brazil, and has been associated to several immunological manifestations such as arthritis, uveitis, dermatitis and Sjögren’s syndrome. This study was intended to evaluate the frequency of autoantibodies in patients infected with HTLV-1 and manifesting keratoconjunctivitis sicca (KCS). HTLV-1 patients with KCS, enrolled in a reference ambulatory of the city of Salvador, were tested for autoantibodies such as antinuclear antibodies, rheumatoid factor, anti-SSA/Ro and anti-SSB/La. Two comparison groups were also included: (a) HTLV-1 patients without KCS and (b) seronegative patients with KCS. Correlation of proviral load (PVL) in HTLV-1 patients with presence or absence of KCS was also assessed. No autoantibodies were detected in HTLV-1 patients with KCS. The PVL of HTLV-1 patients was higher in patients with KCS without other clinical manifestations customarily associated to HTLV-1. In conclusion, in this study, no changes were observed in humoral immunity concerning production of certain autoantibodies in HTLV-1-infected patients with KCS, which suggests that other mechanisms may be involved in the pathogenesis of this manifestation. Additionally, PVL may be a marker of KCS development in these patients.
Similar content being viewed by others
References
Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC (1980) Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA 77(12):7415–7419
Proietti FA, Carneiro-Proietti AB, Catalan-Soares BC, Murphy EL (2005) Global epidemiology of HTLV-I infection and associated diseases. Oncogene 24(39):6058–6068
Galvao-Castro B, Loures L, Rodriques LG et al (1997) Distribution of human T-lymphotropic virus type I among blood donors: a nationwide Brazilian study. Transfusion 37(2):242–243
Dourado I, Alcantara LC, Barreto ML, da Gloria Teixeira M, Galvao-Castro B (2003) HTLV-I in the general population of Salvador, Brazil: a city with African ethnic and sociodemographic characteristics. J Acquir Immune Defic Syndr 34(5):527–531
Yoshida M, Miyoshi I, Hinuma Y (1982) Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci USA 79(6):2031–2035
Gessain A, Barin F, Vernant JC et al (1985) Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet 2(8452):407–410
Nishioka K, Maruyama I, Sato K, Kitajima I, Nakajima Y, Osame M (1989) Chronic inflammatory arthropathy associated with HTLV-I. Lancet 1(8635):441
Ijichi S, Matsuda T, Maruyama I, Ijichi S et al (1990) Arthritis in a human T lymphotropic virus type I (HTLV-I) carrier. Ann Rheum Dis 49(9):718–721
Morgan OS, Rodgers-Johnson P, Mora C, Char G (1989) HTLV-1 and polymyositis in Jamaica. Lancet 2(8673):1184–1187
Eguchi K, Matsuoka N, Ida H et al (1992) Primary Sjogren’s syndrome with antibodies to HTLV-I: clinical and laboratory features. Ann Rheum Dis 51(6):769–776
Terada K, Katamine S, Eguchi K et al (1994) Prevalence of serum and salivary antibodies to HTLV-1 in Sjogren’s syndrome. Lancet 344(8930):1116–1119
Mochizuki M, Watanabe T, Yamaguchi K et al (1992) HTLV-I uveitis: a distinct clinical entity caused by HTLV-I. Jpn J Cancer Res 83(3):236–239
Merle H, Cabre P, Merle S, Gerard M, Smadja D (2001) A description of human T-lymphotropic virus type I-related chronic interstitial keratitis in 20 patients. Am J Ophthalmol 131(3):305–308
Buggage RR, Levy-Clarke GA, Smith JA (2001) New corneal findings in human T-cell lymphotrophic virus type 1 infection. Am J Ophthalmol 131(3):309–313
Merle H, Smadja D, Le Hoang P, Merle H, Smadja D, Le Hoang P et al (1996) Ocular manifestations in patients with HTLV-I associated infection–a clinical study of 93 cases. Jpn J Ophthalmol 40(2):260–270
Merle H, Cabre P, Olindo S, Merle S, Smadja D (2002) Ocular lesions in 200 patients infected by the human T-cell lymphotropic virus type 1 in martinique (French West Indies). Am J Ophthalmol 134(2):190–195
Nakamura H, Eguchi K, Nakamura T et al (1997) High prevalence of Sjogren’s syndrome in patients with HTLV-I associated myelopathy. Ann Rheum Dis 56(3):167–172
Vitali C, Bombardieri S, Moutsopoulos HM et al (1996) Assessment of the European classification criteria for Sjogren’s syndrome in a series of clinically defined cases: results of a prospective multicentre study. The European Study Group on Diagnostic Criteria for Sjogren’s syndrome. Ann Rheum Dis 55(2):116–121
Dehee A, Cesaire R, Desire N et al (2002) Quantitation of HTLV-I proviral load by a TaqMan real-time PCR assay. J Virol Methods 102(1–2):37–51
Green JE, Hinrichs SH, Vogel J, Jay G (1989) Exocrinopathy resembling Sjogren’s syndrome in HTLV-1 tax transgenic mice. Nature 341(6237):72–74
Hida A, Kawabe Y, Kawakami A et al (1999) HTLV-I associated Sjogren’s syndrome is aetiologically distinct from anti-centromere antibodies positive Sjogren’s syndrome. Ann Rheum Dis 58(5):320–322
Nakamura H, Kawakami A, Tominaga M et al (2000) Relationship between Sjogren’s syndrome and human T-lymphotropic virus type I infection: follow-up study of 83 patients. J Lab Clin Med 135(2):139–144
Chisholm DM, Mason DK (1968) Labial salivary gland biopsy in Sjogren’s disease. J Clin Pathol 21(5):656–660
Merle H, Cabre P, Smadja D, Josset P, Landau M, Vernant JC (1999) Sicca syndrome and HTLV-I-associated myelopathy/tropical spastic paraparesis. Jpn J Ophthalmol 43(6):509–512
Pinheiro SR, Lana-Peixoto MA, Proietti AB et al (1995) HTLV-I associated uveitis, myelopathy, rheumatoid arthritis and Sjogren’s syndrome. Arq Neuropsiquiatr 53(4):777–781
Moraes HV Jr, Branco RL, Pinto CMS, Dantas MM, Fiszman R (1995) HTLV-I e Uveíte. Revista Brasileira de Oftalmologia 54(12):39–42
Pinheiro SR, Martins-Filho AO, Ribas JG et al (2006) Immunologic markers, uveitis, and keratoconjunctivitis sicca associated with human T-cell lymphotropic virus type 1. Am J Ophthalmol 142(5):811–815
Giozza SP, Santos SB, Martinelli M, Porto MA, Muniz AL, Carvalho EM (2008) Salivary and lacrymal gland disorders and HTLV-1 infection. Rev Stomatol Chir Maxillofac 109(3):153–157
Santiago M, Crusoé EQ, Matos AV (2002) Manifestações reumatológicas associadas à infecção pelo HTLV-I. Rev Bras Reumatol 42(5):306–310
Ono A, Mochizuki M, Yamaguchi K, Miyata N, Watanabe T (1995) Increased number of circulating HTLV-1 infected cells in peripheral blood mononuclear cells of HTLV-1 uveitis patients: a quantitative polymerase chain reaction study. Br J Ophthalmol 79(3):270–276
Best I, Adaui V, Verdonck K et al (2006) Proviral load and immune markers associated with human T-lymphotropic virus type 1 (HTLV-1)-associated myelopathy/tropical spastic paraparesis (HAM/TSP) in Peru. Clin Exp Immunol 146(2):226–233
Montanheiro PA, Oliveira AC, Posada-Vergara MP et al (2005) Human T-cell lymphotropic virus type I (HTLV-I) proviral DNA viral load among asymptomatic patients and patients with HTLV-I-associated myelopathy/tropical spastic paraparesis. Braz J Med Biol Res 38(11):1643–1647
Acknowledgments
The authors thank Professor Fernanda Grassi for the excellent suggestions provided during the manuscript preparation. MS received a scholarship from CNPq.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferraz-Chaoui, A.K., Atta, A.M., Atta, M.L.S. et al. Study of autoantibodies in patients with keratoconjunctivitis sicca infected by the human T cell lymphotropic virus type 1. Rheumatol Int 30, 775–778 (2010). https://doi.org/10.1007/s00296-009-1066-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-009-1066-5