Abstract
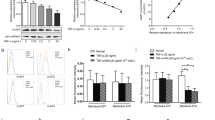
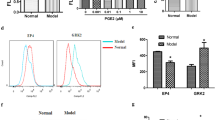
High concentration of epidermal growth factor (EGF) is found in the synovial fluid of rheumatoid arthritis (RA) that might imply the involvement of EGF in the pathogenesis of arthritic diseases. In order to investigate if EGF is involved in the regulation of cyclooxygenase-2 (COX-2) and the prostaglandin E2 (PGE2) production in fibroblast like synoviocytes (FLS) from patients with RA. The levels of COX-2 and microsomal prostaglandin E synthase-1 (mPGES-1) were evaluated using RT-PCR and Western blot analysis. Electrophoretic mobility shift assay (EMSA) was performed to investigate EGF mediated DNA binding activity of nuclear factor-κB (NF-κB). PGE2 levels were analyzed by ELISA. EGF enhanced both COX-2 protein and mRNA expressions. mPGES-1 mRNA level was also increased by EGF treatment. EGF also stimulated ERK1/2 MAPK activity and the inhibition of ERK1/2 by PD098059 (ERK1/2 specific inhibitor) resulted in the suppression of EGF-induced COX-2 expression. The DNA binding activity of NF-κB was remarkably increased by EGF treatment and the pretreatment of PD098059 abolished EGF-stimulated NF-κB activity. We also observed that the level of PGE2 was significantly elevated with the treatment of EGF in FLS, and the pretreatment of PD098059 abolished this stimulating effect. These results suggest that EGF is involved in the inflammatory process of RA by stimulating COX-2 expression and PGE2 production. And EGF enhanced PGE2 production appears to be mediated via ERK1/2 MAPK and NF-κB pathway in FLS.




Similar content being viewed by others
References
Satoh K, Kikuchi S, Sekimata M, Kabuyama Y, Homma MK, Homma Y (2001) Involvement of ErbB-2 in rheumatoid synovial cell growth. Arthritis Rheum 44:260–265. doi:10.1002/1529-0131(200102)44:2<260::AID-ANR42>3.0.CO;2-P
Lui KE, Panchal AS, Santhanagopal A, Dixon SJ, Bernier SM (2002) Epidermal growth factor stimulates proton efflux from chondrocytic cells. J Cell Physiol 192:102–112. doi:10.1002/jcp.10120
Maruotti N, Cantatore FP, Crivellato E, Vacca A, Ribatti D (2006) Angiogenesis in rheumatoid arthritis. Histol Histopathol 21:557–566
Bucala R, Ritchlin C, Winchester R, Cerami A (1991) Constitutive production of inflammatory and mitogenic cytokines by rheumatoid synovial fibroblasts. J Exp Med 173:569–574. doi:10.1084/jem.173.3.569
Brinckerhoff CE (1983) Morphologic and mitogenic responses of rabbit synovial fibroblasts to transforming growth factor beta require transforming growth factor alpha or epidermal growth factor. Arthritis Rheum 26:1370–1379. doi:10.1002/art.1780261110
Amin DN, Hida K, Bielenberg DR, Klagsbrun M (2006) Tumor endothelial cells express epidermal growth factor receptor (EGFR) but not ErbB3 and are responsive to EGF and to EGFR kinase inhibitors. Cancer Res 66:2173–2180. doi:10.1158/0008-5472.CAN-05-3387
Krampera M, Pasini A, Rigo A, Scupoli MT, Tecchio C, Malpeli G, Scarpa A, Dazzi F, Pizzolo G, Vinante F (2005) HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: inducing cell expansion and reversibly preventing multilineage differentiation. Blood 106:59–66. doi:10.1182/blood-2004-09-3645
Ongusaha PP, Kwak JC, Zwible AJ, Macip S, Higashiyama S, Taniguchi N, Fang L, Lee SW (2004) HB-EGF is a potent inducer of tumor growth and angiogenesis. Cancer Res 64:5283–5290. doi:10.1158/0008-5472.CAN-04-0925
Xu YH, Richert N, Ito S, Merlino GT, Pastan I (1984) Characterization of epidermal growth factor receptor gene expression in malignant and normal human cell lines. Proc Natl Acad Sci USA 81:7308–7312. doi:10.1073/pnas.81.23.7308
Klijn JG, Berns PM, Schmitz PI, Foekens JA (1992) The clinical significance of epidermal growth factor receptor (EGF-R) in human breast cancer: a review on 5232 patients. Endocr Rev 13:3–17
Chien PS, Mak OT, Huang HJ (2006) Induction of COX-2 protein expression by vanadate in A549 human lung carcinoma cell line through EGF receptor and p38 MAPK-mediated pathway. Biochem Biophys Res Commun 339:562–568. doi:10.1016/j.bbrc.2005.11.045
Wu W, Silbajoris RA, Whang YE, Graves LM, Bromberg PA, Samet JM (2005) p38 and EGF receptor kinase-mediated activation of the phosphatidylinositol 3-kinase/Akt pathway is required for Zn2+-induced cyclooxygenase-2 expression. Am J Physiol Lung Cell Mol Physiol 289:L883–L889. doi:10.1152/ajplung.00197.2005
Flower RJ (2003) The development of COX2 inhibitors. Nat Rev 2:179–191
Egg D (1984) Concentrations of prostaglandins D2, E2, F2 alpha, 6-keto-F1 alpha and thromboxane B2 in synovial fluid from patients with inflammatory joint disorders and osteoarthritis. Z Rheumatol 43:89–96
Silman AJ (1988) The 1987 revised American Rheumatism Association criteria for rheumatoid arthritis. Br J Rheumatol 27:341–343. doi:10.1093/rheumatology/27.5.341
Du J, Jiang B, Barnard J (2005) Differential regulation of cyclooxygenase-2 in nontransformed and ras-transformed intestinal epithelial cells. Neoplasia 7:761–770. doi:10.1593/neo.04652
Han C, Wu T (2005) Cyclooxygenase-2-derived prostaglandin E2 promotes human cholangiocarcinoma cell growth and invasion through EP1 receptor-mediated activation of the epidermal growth factor receptor and Akt. J Biol Chem 280:24053–24063. doi:10.1074/jbc.M500562200
Johnson FM, Yang P, Newman RA, Donato NJ (2004) Cyclooxygenase-2 induction and prostaglandin E2 accumulation in squamous cell carcinoma as a consequence of epidermal growth factor receptor activation by imatinib mesylate. J Exp Ther Oncol 4:317–325
Slice LW, Chiu T, Rozengurt E (2005) Angiotensin II and epidermal growth factor induce cyclooxygenase-2 expression in intestinal epithelial cells through small GTPases using distinct signaling pathways. J Biol Chem 280:1582–1593. doi:10.1074/jbc.M408172200
Gupta RA, Dubois RN (2001) Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer 1:11–21. doi:10.1038/35094017
Charles P, Elliott MJ, Davis D, Potter A, Kalden JR, Antoni C, Breedveld FC, Smolen JS, Eberl G, de Woody K et al (1999) Regulation of cytokines, cytokine inhibitors, and acute-phase proteins following anti-TNF-alpha therapy in rheumatoid arthritis. J Immunol 163:1521–1528
Siegle I, Klein T, Backman JT, Saal JG, Nusing RM, Fritz P (1998) Expression of cyclooxygenase 1 and cyclooxygenase 2 in human synovial tissue: differential elevation of cyclooxygenase 2 in inflammatory joint diseases. Arthritis Rheum 41:122–129. doi:10.1002/1529-0131(199801)41:1<122::AID-ART15>3.0.CO;2-8
Huber LC, Künzler P, Boyce SH, Michel BA, Gay RE, Ink BS, Gay S (2008) Effects of a novel tyrosine kinase inhibitor in rheumatoid arthritis synovial fibroblasts. Ann Rheum Dis 67:389–394. doi:10.1136/ard.2007.072330
Jakobsson PJ, Thoren S, Morgenstern R, Samuelsson B (1999) Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc Natl Acad Sci USA 96:7220–7225. doi:10.1073/pnas.96.13.7220
Thoren S, Weinander R, Saha S, Jegerschold C, Pettersson PL, Samuelsson B, Hebert H, Hamberg M, Morgenstern R, Jakobsson PJ (2003) Human microsomal prostaglandin E synthase-1: purification, functional characterization, and projection structure determination. J Biol Chem 278:22199–22209. doi:10.1074/jbc.M303227200
Westman M, Korotkova M, af Klint E, Stark A, Audoly LP, Klareskog L, Ulfgren AK, Jakobsson PJ (2004) Expression of microsomal prostaglandin E synthase 1 in rheumatoid arthritis synovium. Arthritis Rheum 50:1774–1780. doi:10.1002/art.20286
Dean JL, Sarsfield SJ, Tsounakou E, Saklatvala J (2003) p38 Mitogen-activated protein kinase stabilizes mRNAs that contain cyclooxygenase-2 and tumor necrosis factor AU-rich elements by inhibiting deadenylation. J Biol Chem 278:39470–39476. doi:10.1074/jbc.M306345200
LaPointe MC, Isenovic E (1999) Interleukin-1beta regulation of inducible nitric oxide synthase and cyclooxygenase-2 involves the p42/44 and p38 MAPK signaling pathways in cardiac myocytes. Hypertension 33:276–282
Lasa M, Mahtani KR, Finch A, Brewer G, Saklatvala J, Clark AR (2000) Regulation of cyclooxygenase 2 mRNA stability by the mitogen-activated protein kinase p38 signaling cascade. Mol Cell Biol 20:4265–4274. doi:10.1128/MCB.20.12.4265-4274.2000
Faour WH, He Y, He QW, de Ladurantaye M, Quintero M, Mancini A, Di Battista JA (2001) Prostaglandin E(2) regulates the level and stability of cyclooxygenase-2 mRNA through activation of p38 mitogen-activated protein kinase in interleukin-1 beta-treated human synovial fibroblasts. J Biol Chem 276:31720–31731. doi:10.1074/jbc.M104036200
Sheng H, Williams CS, Shao J, Liang P, DuBois RN, Beauchamp RD (1998) Induction of cyclooxygenase-2 by activated Ha-ras oncogene in Rat-1 fibroblasts and the role of mitogen-activated protein kinase pathway. J Biol Chem 273:22120–22127. doi:10.1074/jbc.273.34.22120
Matsuura H, Sakaue M, Subbaramaiah K, Kamitani H, Eling TE, Dannenberg AJ, Tanabe T, Inoue H, Arata J, Jetten AM (1999) Regulation of cyclooxygenase-2 by interferon gamma and transforming growth factor alpha in normal human epidermal keratinocytes and squamous carcinoma cells. Role of mitogen-activated protein kinases. J Biol Chem 274:29138–29148. doi:10.1074/jbc.274.41.29138
Guan Z, Buckman SY, Miller BW, Springer LD, Morrison AR (1998) Interleukin-1beta-induced cyclooxygenase-2 expression requires activation of both c-Jun NH2-terminal kinase and p38 MAPK signal pathways in rat renal mesangial cells. J Biol Chem 273:28670–28676. doi:10.1074/jbc.273.44.28670
Tsai SH, Liang YC, Chen L, Ho FM, Hsieh MS, Lin JK (2002) Arsenite stimulates cyclooxygenase-2 expression through activating IkappaB kinase and nuclear factor kappaB in primary and ECV304 endothelial cells. J Cell Biochem 84:750–758. doi:10.1002/jcb.10096
Syeda F, Grosjean J, Houliston RA, Keogh RJ, Carter TD, Paleolog E, Wheeler-Jones CP (2006) Cyclooxygenase-2 induction and prostacyclin release by protease-activated receptors in endothelial cells require cooperation between mitogen-activated protein kinase and NF-kappaB pathways. J Biol Chem 281:11792–11804. doi:10.1074/jbc.M509292200
Author information
Authors and Affiliations
Corresponding author
Additional information
Seong-Su Nah and Hye-Jin Won have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Nah, SS., Won, HJ., Ha, E. et al. Epidermal growth factor increases prostaglandin E2 production via ERK1/2 MAPK and NF-κB pathway in fibroblast like synoviocytes from patients with rheumatoid arthritis. Rheumatol Int 30, 443–449 (2010). https://doi.org/10.1007/s00296-009-0976-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-009-0976-6