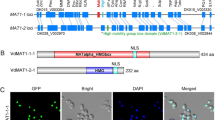
Abstract
Rice false smut caused by Villosiclava virens is one of the destructive diseases on panicles of rice. Sexual development of V. virens, controlled by mating-type locus, plays an important role in the prevalence of rice false smut and genetic diversity of the pathogen. However, how the mating-type genes mediate sexual development of the V. virens remains largely unknown. In this study, we characterized the two mating-type genes, MAT1-1-1 and MAT1-1-2, in V. virens. MAT1-1-1 knockout mutant showed defects in hyphal growth, conidia morphogenesis, sexual development, and increase in the tolerance to salt and osmotic stress. Targeted deletion of MAT1-1-2 not only impaired the sclerotia formation and pathogenicity of V. virens, but also reduced the production of conidia. The MAT1-1-2 mutant showed increases in tolerance to salt and hydrogen peroxide stress, but decreases in tolerance to osmotic stress. Yeast two-hybrid assay showed that MAT1-1-1 interacted with MAT1-1-2, indicating that those proteins might form a complex to regulate sexual development. In addition, MAT1-1-1 localized in the nucleus, and MAT1-1-2 localized in the cytoplasm. Collectively, our results demonstrate that MAT1-1-1 and MAT1-1-2 play important roles in the conidiation, stress response, sexual development, and pathogenicity of V. virens, thus providing new insights into the function of mating-type gene.







Similar content being viewed by others
References
Arnaise S, Debuchy R, Picard M (1997) What is a Bona fide mating-type gene? Internuclear complementation of mat mutants in Podospora anserina. Mol Gen Genet 256:169–178
Bobrowicz P, Pawlak R, Correa A, Bell-Pedersen D, Ebbole DJ (2002) The Neurospora crassa pheromone precursor genes are regulated by the mating type locus and circadian clock. Mol Microbiol 45:795–804
Böhm J, Hoff B, O’Gorman CM, Wolfers S, Klix V, Binger D, Zadra I, Kürnsteiner H, Pöggeler S, Dyer PS, Kück U (2013) Sexual reproduction and mating-type-mediated strain development in the penicillin-producing fungus Penicillium chrysogenum. PNAS 110:1476–1481
Coppin E, Debuchy R, Arnaise S, Picard M (1997) Mating types and sexual development in filamentous ascomycetes. Microbiol Mol Biol Rev 61:411–428
Coppin E, de Renty C, Debuchy R (2005) The function of the coding sequences for the putative pheromone precursors in Podospora anserina is restricted to fertilization. Eukaryot Cell 4:407–420
David-Palma M, Sampaio JP, Gonçalves P (2016) Genetic dissection of sexual reproduction in a primary homothallic basidiomycete. PLoS Genet 12:e1006110
Debuchy R, Turgeon BG (2006) Mating-type structure, evolution, and function in euascomycetes. In: Kües U, Fischer R (eds) The mycota, vol 1. Growth, differentiation and sexuality. Springer, Berlin, pp 293–323
Debuchy R, Berteaux-Leceleir V, Silar P (2010) Mating systems and sexual morphogenesis in ascomycetes. In: Borkovich KA, Ebbole DJ (eds) Cellular and molecular biology of filamentous fungi. ASM Press, Washington, DC, pp 501–535
Doughan B, Rollins JA (2016) Characterization of MAT gene functions in the life cycle of Sclerotinia sclerotiorum reveals a lineage-specific MAT gene functioning in apothecium morphogenesis. Fungal Biol 120:1105–1117
Ene I, Bennett R (2014) The cryptic sexual strategies of human fungal pathogens. Nat Rev Microbiol 12:239–251
Fan J, Guo XY, Li L, Huang F, Sun WX, Li Y, Huang YY, Xu YJ, Shi J, Lei Y, Zheng AP, Wang WM (2015) Infection of Ustilaginoidea virens intercepts rice seed formation but activates grain-filling-related genes. J Integr Plant Biol 57:577–590
Fan J, Yang J, Wang YQ, Li GB, Li Y, Huang F, Wang WM (2016a) Current understanding on Villosiclava virens, a unique flower-infecting fungus causing rice false smut disease. Mol Plant Pathol 17:1321–1330
Fan LL, Yong ML, Li DY, Liu YJ, Lai CH, Chen HM, Cheng FM, Hu DW (2016b) Effect of temperature on the development of sclerotia in Villosiclava virens. J Integr Agric 15:2550–2555
Fang AF, Gao H, Zhang N, Zheng XH, Qiu SS, Li YJ, Zhou S, Cui FH, Sun WX (2019) A novel effector gene SCRE2 contributes to full virulence of Ustilaginoidea virens to rice. Front Microbiol 10:845
Ferreira AV, Saupe S, Glass NL (1996) Transcriptional analysis of the mtA idiomorph of Neurospora crassa identifies two genes in addition to mtA-1. Mol Gen Genet 250:767–774
Ferreira AV, An Z, Metzenberg RL, Glass NL (1998) Characterization of mat A-2, mat A-3 and ∆matA mating-type mutants of Neurospora crassa. Genetics 148:1069–1079
Fraser JA, Heitman J (2005) Chromosomal sex-determining regions in animals, plants and fungi. Curr Opin Genet Dev 15:645–651
Fu X, Xie R, Wang J, Chen X, Wang X, Sun W, Meng J, Lai D, Zhou L, Wang B (2017) Development of colloidal gold-based lateral flow immunoassay for rapid qualitative and semiquantitative analysis of ustiloxins A and B in rice samples. Toxins (Basel) 9:79
Glass NL, Lee L (1992) Isolation of Neurospora crassa A mating type mutants by repeat induced point (Rip) mutation. Genetics 132:125–133
Haber JE (2012) Mating-type genes and MAT switching in Saccharomyces cerevisiae. Genetics 191:33–64
Harari Y, Ram Y, Kupiec M (2018) Frequent ploidy changes in growing yeast cultures. Curr Genet 64:1001–1004
Hu ML, Luo LX, Wang S, Liu YF, Li JQ (2014) Infection processes of Ustilaginoidea virens during artificial inoculation of rice panicles. Eur J Plant Pathol 139:67–77
Jecmen AC, Tebeest DO (2015) First report of the occurrence of a white smut infecting rice in Arkansas. J Phytopathol 163:138–143
Jones SK Jr, Bennett RJ (2011) Fungal mating pheromones: choreographing the dating game. Fungal Genet Biol 48:668–676
Kämper J, Reichmann M, Romeis T, Bolker M, Kahmann R (1995) Multiallelic recognition: nonself-dependent dimerization of the bE and bW homeodomain proteins in Ustilago maydis. Cell 81:73–83
Kim HK, Lee T, Yun SH (2008) A putative pheromone signaling pathway is dispensable for self-fertility in the homothallic ascomycete Gibberella zeae. Fungal Genet Biol 45:1188–1196
Klix V, Nowrousian M, Ringelberg C, Loros JJ, Dunlap JC, Pöggeler S (2010) Functional characterization of MAT1-1-specifc mating-type genes in the homothallic ascomycete Sordaria macrospora provides new insights into essential and nonessential sexual regulators. Eukaryot Cell 9:894–905
Kües U, James T, Heitman J (2011) 6 Mating type in Basidiomycetes: unipolar, bipolar, and tetrapolar patterns of sexuality. In: Pöggeler S, Wöstemeyer J (eds) Evolution of fungi and fungal-like organisms. Springer, Berlin, pp 97–160
Ladhalakshmi D, Laha GS, Singh R, Karthikeyan A, Mangrauthia SK, Sundaram RM, Thukkaiyannan P, Viraktamath BC (2012) Isolation and characterization of Ustilaginoidea virens and survey of false smut disease of rice in India. Phytoparasitica 40:171–176
Lee SC, Ni M, Li WJ, Cecelia S, Heitman J (2010) The evolution of sex: a perspective from the fungal kingdom. Microbiol Mol Biol Rev 74:298–340
Li PX, Evans CD, Wu YZ, Cao B, Hamel E, Joullié MM (2008) Evolution of the total syntheses of ustiloxin natural products and their analogues. J Am Chem Soc 130:2351–2364
Li YJ, Wang M, Liu ZH, Zhang K, Cui FH, Sun WX (2019) Towards understanding the biosynthetic pathway for ustilaginoidin mycotoxins in Ustilaginoidea virens. Environ Microbiol 21:2629–2643
Liang YF, Han Y, Wang CF, Jiang C, Xu JR (2018) Targeted deletion of the USTA and UvSLT2 genes efficiently in Ustilaginoidea virens with the CRISPR-Cas9 System. Front Plant Sci 9:699
Liu Y, Liu JK, Li GH, Zhang MZ, Zhang YY, Wang YY, Hou J, Yang S, Sun J, Qin QM (2019) A novel Botrytis cinerea-specific gene BcHBF1 enhances virulence of the grey mould fungus via promoting host penetration and invasive hyphal development. Mol Plant Pathol 20:731–747
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔCt method. Methods 25:402–408
Lu YZ, Xia YL, Luo FF, Dong CH, Wang CS (2016) Functional convergence and divergence of mating-type genes fulfilling in Cordyceps militaris. Fungal Genet Biol 88:35–43
Martin T, Lu SW, van Tilbeurgh H, Ripoll DR, Dixelius C, Turgeon BG, Debuchy R (2010) Tracing the origin of the fungal α1 domain places its ancestor in the HMG-box superfamily: implication for fungal mating-type evolution. PLoS ONE 5:e15199
Paoletti M, Seymour FA, Alcocer MJC, Kaur N, Calvo AM, Archer DB, Dyer PS (2007) Mating type and the genetic basis of self-fertility in the model fungus Aspergillus nidulans. Curr Biol 17:1384–1389
Pöggeler S (2000) Two pheromone precursor genes are transcriptionally expressed in the homothallic ascomycete Sordaria macrospora. Curr Genet 37:403–411
Pöggeler S, Kück U (2001) Identification of transcriptionally expressed pheromone receptor genes in filamentous ascomycetes. Gene 280:9–17
Rodenburg SYA, Terhem RB, Veloso J, Stassen JHM, van Kan JAL (2018) Functional analysis of mating type genes and transcriptome analysis during fruiting body development of Botrytis cinerea. mBio 9:e01939-17
Roncero C, Durán A (1985) Effect of calcofluor white and congo red on fungal cell wall morphogenesis: in vivo activation of chitin polymerization. J Bacteriol 163:1180–1185
Shiu PK, Glass NL (2000) Cell and nuclear recognition mechanisms mediated by mating type in filamentous ascomycetes. Curr Opin Microbiol 3:183–188
Snetselaar KM, Bölker M, Kahmann R (1996) Ustilago maydis mating hyphae orient their growth toward pheromone sources. Fungal Genet Biol 20:299–312
Song JH, Wei W, Lv B, Lin Y, Yin WX, Peng YL, Schnabel G, Huang JB, Jiang DH, Luo CX (2016) Rice false smut fungus hijacks the rice nutrients supply by blocking and mimicking the fertilization of rice ovary. Environ Microbiol 18:3840–3849
Spellig T, Bölker M, Lottspeich F, Frank RW, Kahmann R (1994) Pheromones trigger filamentous growth in Ustilago maydis. EMBO J 13:1620–1627
Su X, Rehman L, Guo H, Li X, Cheng H (2018) The oligosaccharyl transferase subunit STT3 mediates fungal development and is required for virulence in Verticillium dahlia. Curr Genet 64:235–246
Szewczyk E, Krappmann S (2010) Conserved regulators of mating are essential for Aspergillus fumigatus cleistothecium formation. Eukaryot Cell 9:774–783
Tang YX, Jin J, Hu DW, Yong ML, Xu Y, He LP (2013) Elucidation of the infection process of Ustilaginoidea virens (teleomorph: Villosiclava virens) in rice spikelets. Plant Pathol 62:1–8
Tang J, Bai J, Chen X, Zheng L, Liu H, Huang J (2020) Two protein kinases UvPmk1 and UvCDC2 with significant functions in conidiation, stress response and pathogenicity of rice false smut fungus Ustilaginoidea virens. Curr Genet 66(2):409–420
Thon G, Maki T, Haber JE, Iwasaki H (2019) Mating-type switching by homology-directed recombinational repair: a matter of choice. Curr Genet 65:351–362
Turgeon BG, Debuchy R (2007) Cochliobolus and Podospora: mechanisms of sex determination and the evolution of reproductive lifestyle. In: Heitman J, Kronstad JW, Taylor JW, Casselton L (eds) Sex in fungi. ASM Press, Washington, DC, pp 93–121
Turgeon BG, Yoder OC (2000) Proposed nomenclature for mating type genes of filamentous ascomycetes. Fungal Genet Biol 31:1–5
Turgeon BG, Christiansen SK, Yoder OC (1993) Mating type genes in ascomycetes and their imperfect relatives. In: Reynolds DR, Taylor JW (eds) The fungal holomorph: mitotic, meiotic, and pleomorphic speciation in fungal systematics. CAB International, Wallingford, pp 199–215
Verna J, Ballester R (1999) A novel role for the mating type MAT locus in the maintenance of cell wall integrity in Saccharomyces cerevisiae. Mol Gen Genet 261:681–689
Wang Q, Wang S, Xiong CL, James TY, Zhang XG (2017a) Mating-type genes of the anamorphic fungus Ulocladium botrytis affect both asexual sporulation and sexual reproduction. Sci Rep 7:7932
Wang X, Wang J, Lai D, Wang W, Dai J, Zhou L, Liu Y (2017b) Ustiloxin G, a new cyclopeptide mycotoxin from rice false smut balls. Toxins (Basel) 9:54. https://doi.org/10.3390/toxins9020054
Weigel D, Glazebrook J (2006) Transformation of Agrobacterium using the freeze-thaw method. CSH Protoc 7:1031–1036
Wilken PM, Steenkamp ET, Wingfield MJ, De Beer ZW, Wingfield BD (2017) Which MAT gene? Pezizomycotina (Ascomycota) mating-type gene nomenclature reconsidered. Fungal Biol Rev 31:199–211
Xie SL, Wang YF, Wei W, Li CY, Liu Y, Qu JS, Meng QH, Lin Y, Yin WX, Yang YN, Luo CX (2019) The Bax inhibitor UvBI-1, a negative regulator of mycelial growth and conidiation, mediates stress response and is critical for pathogenicity of the rice false smut fungus Ustilaginoidea virens. Curr Genet 65:1185–1197
Yong ML, Deng QD, Fan LL, Miao JK, Lai CH, Chen HM, Yang XJ, Wang S, Chen FR, Jin L, Yang BH, Bai YJ, Wang ZY, Hu DW (2018) The role of Ustilaginoidea virens sclerotia in increasing incidence of rice false smut disease in the subtropical zone in China. Eur J Plant Pathol 150:669–677
Yu JJ, Sun WX, Yu MN, Yin XL, Meng XK, Zhao J, Huang L, Huang L, Liu YF (2015a) Characterization of mating-type loci in rice false smut fungus Villosiclava virens. FEMS Microbiol Lett 362:fnv014
Yu MN, Yu JJ, Hu JK, Huang L, Wang YH, Yin XL, Nie YF, Meng XK, Wang WD, Liu YF (2015b) Identification of pathogenicity-related genes in the rice pathogen Ustilaginoidea virens through random insertional mutagenesis. Fungal Genet Biol 76:10–19
Yu JJ, Yu MN, Nie YF, Sun WX, Yin XL, Zhao J, Wang YH, Ding H, Qi ZQ, Du Y, Huang L, Liu YF (2016) Comparative transcriptome analysis of fruiting body and sporulating mycelia of Villosiclava virens reveals genes with putative functions in sexual reproduction. Curr Genet 62:575–584
Yu JJ, Yu MN, Song TQ, Cao HJ, Pan XY, Yong ML, Qi ZQ, Du Y, Zhang RS, Yin XL, Liu YF (2019) A homeobox transcription factor UvHOX2 regulates chlamydospore formation, conidiogenesis, and pathogenicity in Ustilaginoidea virens. Front Microbiol 10:1071. https://doi.org/10.3389/fmicb.2019.01071
Zhang Y, Zhang K, Fang AF, Han YQ, Yang J, Xue MF, Bao JD, Hu DW, Zhou B, Sun XY, Li SJ, Wen M, Yao N, Ma LJ, Liu YF, Zhang M, Huang F, Luo CX, Zhou LG, Li JQ, Chen ZY, Miao JK, Wang S, Lai JS, Xu JR, Hsiang T, Peng YL, Sun WX (2014) Specific adaptation of Ustilaginoidea virens in occupying host florets revealed by comparative and functional genomics. Nat Commun 5:3849. https://doi.org/10.1038/ncomms4849
Zheng P, Wang CS (2013) Sexuality control and sex evolution in fungi. Scientia Sinica Vitae 43:1090–1097
Zheng Q, Hou R, Zhang JY, Ma JW, Wu ZS, Wang GH, Wang CF, Xu JR (2015) The MAT locus genes play different roles in sexual reproduction and pathogenesis in Fusarium graminearum. PLoS ONE 10:e0131623
Acknowledgements
We thank JinRong Xu (Purdue University, USA) for providing the CRISPR-Cas9 system plasmid. This work was supported by the National Natural Science Foundation of China (No. 31571961) and the China Postdoctoral Science Foundation (2018M632257).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by M. Kupiec.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yong, M., Yu, J., Pan, X. et al. Two mating-type genes MAT1-1-1 and MAT1-1-2 with significant functions in conidiation, stress response, sexual development, and pathogenicity of rice false smut fungus Villosiclava virens. Curr Genet 66, 989–1002 (2020). https://doi.org/10.1007/s00294-020-01085-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-020-01085-9