Abstract
Autoregulation is the direct modulation of gene expression by the product of the corresponding gene. Autoregulation of bacterial gene expression has been mostly studied at the transcriptional level, when a protein acts as the cognate transcriptional repressor. A recent study investigating dynamics of the bacterial toxin–antitoxin MazEF system has shown how autoregulation at both the transcriptional and post-transcriptional levels affects the heterogeneity of Escherichia coli populations. Toxin–antitoxin systems hold a crucial but still elusive part in bacterial response to stress. This perspective highlights how these modules can also serve as a great model system for investigating basic concepts in gene regulation. However, as the genomic background and environmental conditions substantially influence toxin activation, it is important to study (auto)regulation of toxin–antitoxin systems in well-defined setups as well as in conditions that resemble the environmental niche.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Overview of toxin–antitoxin systems
Toxin–antitoxin (TA) modules are highly versatile systems, generally widespread in prokaryotic genomes (Gerdes et al. 2005; Van Melderen 2010; Yamaguchi and Inouye 2011; Gerdes 2012; Rocker and Meinhart 2016; Hõrak and Tamman 2017). Toxin activation during adverse conditions inhibits fundamental cellular processes, such as replication, translation, and cell wall synthesis, thereby reducing bacterial growth. TA systems are categorized into at least six different types, depending on how the antitoxin neutralizes expression and/or activity of the toxin (Harms et al. 2018). The model Escherichia coli K-12 MG1655 strain has at least 37 TA loci in total, with at least 10 toxins characterized as type II toxins with RNA-degrading activity, i.e., endoribonucleases. Type II endoribonucleases can be active independent of ribosomes or in a ribosome-dependent manner, and few of them cleave RNA sequence specifically (Masuda and Inouye 2017; Harms et al. 2018). Stressful conditions promote the activity of the Clp and Lon proteases that degrade type II antitoxins (Goeders and Van Melderen 2014).
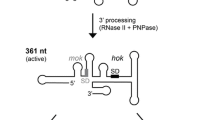
In general, the MazEF system is one of the most studied type II TA modules. The MazE antitoxin directly binds to the MazF toxin and forms a stable complex thereby neutralizing the MazF activity (Kamada et al. 2003). It has been biochemically shown that the MazE antitoxin is degraded by ClpAP (Aizenman et al. 1996), while the Lon protease has additionally been implicated in the mazEF regulation (Christensen et al. 2003; Tripathi et al. 2014). Upon MazE proteolysis, the MazF toxin is liberated from the complex, and degrades single-stranded RNA at specific sequences. MazF of E. coli recognizes an ACA sequence as the core cleavage site (Zhang et al. 2003); however, specific nucleotides flanking the core sequence, as well as the RNA secondary structure contribute to the efficiency of MazF-mediated RNA cleavage (Culviner and Laub 2018).
Transcriptional autoregulation of TA systems
Transcriptional autoregulation has been biochemically described for many type II TA systems, usually through the mechanism of conditional cooperativity (Overgaard et al. 2008; Garcia-Pino et al. 2010). This mechanism defines the state of transcription given the molar ratio of toxin to antitoxin within a cell (Page and Peti 2016). When the ratio is similar, the toxin acts as a co-repressor within the toxin–antitoxin complex, and the complex strongly represses transcription. When the toxin to antitoxin ratio is in favor of the antitoxin, the antitoxin alone acts a weaker transcriptional repressor. In conditions that promote degradation of the antitoxin, toxin to antitoxin ratio is in favor of the toxin, which leads to the de-repression of transcription of the TA module (Gelens et al. 2013; Cataudella et al. 2013; Vandervelde et al. 2016).
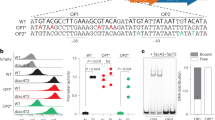
Conditional cooperativity has also been suggested for the autoregulation of mazEF expression (Zorzini et al. 2015). During exponential growth, the expression of the mazEF module is strongly repressed (Marianovsky et al. 2001; Nikolic et al. 2017). Analysis of the mazEF promoter activity in a mazF deletion strain, when the toxin to antitoxin ratio is artificially shifted in favor of the MazE antitoxin, shows weak repression of the mazEF operon. Adverse conditions promote modest transcriptional activation and potential de-repression of the mazEF module (Muthuramalingam et al. 2016; Shan et al. 2017; Nikolic et al. 2017), suggesting transient de novo synthesis of the toxin and antitoxin proteins at low levels. As de-repression has been directly shown only for high toxin to antitoxin ratios that are seemingly not measured in vivo, a mechanistic explanation of possible conditional cooperativity in the mazEF regulation is still elusive (Zorzini et al. 2015).
Post-transcriptional autoregulation of TA systems
RNA-based regulation of TA systems has been mostly described for type I toxins (Page and Peti 2016; Berghoff et al. 2017; Berghoff and Wagner 2017). However, type II TA systems can also be regulated at the RNA level, as recently shown in the case of post-transcriptional autoregulation of mazEF expression (Nikolic et al. 2018). During ectopic mazF expression, the MazF toxin cleaves the mazF mRNA at ACA sites, which has direct consequences on the behavior of single cells. Single-cell analysis indicated that the cell length fluctuates substantially during conditions that promote MazF activation. Cells elongate and become filamentous, and divide irregularly during mazF overexpression, which suggests that mRNAs of proteins involved in cell elongation and division are targeted by MazF (Schifano et al. 2014; Sauert et al. 2016; Venturelli et al. 2017). A mathematical model showed that MazF-mediated cleavage of the mazEF mRNA can result in stochastic pulsed excitations of MazF levels in single cells, suggesting that the observed cell length fluctuations are a consequence of the fluctuations in MazF levels during stress. Further analysis indicated that the frequency of the toxin spikes and the amount of free toxin released during the pulses depend on the amount of stress (Vet, Vandervelde and Gelens, unpublished). Overall, the majority of the known type II TA systems are RNA-degrading enzymes, degrading RNA while bound to the ribosome, or in a ribosome-independent manner, such as the MazF toxin (Masuda and Inouye 2017; Harms et al. 2018). Thus, an open question remains: Do other RNA-degrading toxins also autoregulate their expression by degrading their cognate mRNAs? And more importantly, how does post-transcriptional autoregulation of type II TA systems influence the fate of bacterial cells in stressful conditions, for instance during antibiotic treatment or nutrient starvation?
Autoregulatory circuits and feedback loops in TA systems
In a one-player negative feedback system, a protein represses its own expression. One prominent example is the regulation of tetR expression by the TetR transcriptional repressor (Becskei and Serrano 2000; Rosenfeld et al. 2002). Autoregulation of the mazEF expression contains negative and positive feedback. Negative feedback manifests when the MazE–MazF complex represses transcription of the operon (Marianovsky et al. 2001). At the post-transcriptional level, complete mazEF mRNA degradation by MazF prevents de novo synthesis of the toxin and antitoxin proteins, and favors long-lived MazF toxin over short-lived MazE antitoxin, thus resulting in a positive feedback loop (Nikolic et al. 2018). Another recent model has analyzed TA regulatory circuits in a setting that can be generally applied to any stressful condition that results in growth reduction and antitoxin degradation (Tian et al. 2017). The modeled TA locus, which consists of a toxin gene downstream of an antitoxin gene, is transcribed into a bicistronic mRNA. Toxin-mediated cleavage of the toxin part of the mRNA leads to the antitoxin protein synthesis, thus manifesting as a negative feedback. As the toxin module in this model contains no ribosome binding site, cleavage in the antitoxin part of the mRNA prevents synthesis of both antitoxin and toxin proteins, thus manifesting as a positive feedback (Tian et al. 2017). In general, the MazEF system contains at least two players and several interactions, thus feedback loops possibly operating at different levels and different time scales. Distinct regulatory circuits within this TA system can be monitored in a synthetic experimental setup by adjusting levels of toxin and antitoxin production.
Genomic background and environmental cues as determinants of TA regulation
Even though there is substantial information about TA systems in non-pathogenic, laboratory strains of E. coli, less is known about TA systems in pathogenic E. coli. One reason for this lack of knowledge is that TA loci are acquired through horizontal gene transfer, and thus are not conserved among different isolates belonging to the same bacterial species (Fiedoruk et al. 2015; Ramisetty and Santhosh 2015); for instance, the mazEF locus is present in the non-pathogenic K-12 MG1655 E. coli strain, but not in the uropathogenic CFT073 strain (Norton and Mulvey 2012). Moreover, the underlying genomic background can shape the response of individual TA systems during stress. Because TA systems form complex regulatory networks by cross-activating each other (Yamaguchi and Inouye 2011; Kasari et al. 2013; Wessner et al. 2015), it is important to study the activity of individual toxins given the strain-specific genomic background. Sequence-identical toxin homologs from different strains could thus differ in their efficacy and the time needed for their activation.
Besides contribution of genomic background to regulation and autoregulation of TA operons, another factor is crucial when investigating TA systems—environmental signals (Ramisetty and Santhosh 2017; Hõrak and Tamman 2017). Based on analysis of a commonly used laboratory strain, deletion of a single TA locus does not influence competiveness of E. coli populations in a culture flask (Tsilibaris et al. 2007). However, a previous study has shown that individual TA systems of an uropathogenic E. coli strain indeed provide growth advantage during niche-specific colonization (Norton and Mulvey 2012). Furthermore, TA modules of Salmonella promote formation of intracellular persisters upon phagocytosis by macrophages (Helaine et al. 2014). These studies highlight the strong need to investigate the regulation and roles of TA systems in pathogenic bacteria during infection, as bacteria do not experience cues from their environmental niches while growing in the laboratory medium. Little is known about signals that trigger TA systems in general; however, it is assumed that TA systems respond to stressful conditions (Aizenman et al. 1996; Ramisetty and Santhosh 2017; Harms et al. 2018; Goormaghtigh et al. 2018). An essential question still remains: What are actual environmental signals that promote activation of TA systems in the bacterial natural niches?
Experimental setups for analysis of TA systems
As genomic background and environmental cues are determinants of TA regulation, it is important to pay attention to the strain genotype and to note exact conditions of laboratory cultivation when investigating TA (auto)regulation in commonly used E. coli laboratory strains. When planning the experimental setup, one needs to consider several aspects, for instance, to carefully decide on which E. coli laboratory strain to use: the MG1655 and MC4100 strains differ from each other significantly in their genotypes (Peters et al. 2003; Kolodkin-Gal and Engelberg-Kulka 2008) (e.g., compare Vesper et al. 2011 with Culviner and Laub 2018). Experiments can have different outcomes depending on whether the chosen strain has a functional relA locus (Tsilibaris et al. 2007), or if the strain carries prophage insertions or other genomic rearrangements that potentially influence the TA response (Harms et al. 2017; Goormaghtigh et al. 2018). Furthermore, different ectopic expression systems can produce different toxin levels based on the strength of the inducible promoter, as well as whether the expression system is inserted into the chromosome, or based on a medium-copy or a high-copy plasmid. For instance, mild ectopic mazF expression results in slight growth reduction (Nikolic et al. 2017), while ectopic mazF overexpression from a medium-copy plasmid results in stronger growth reduction and promotes formation of phenotypically distinct subpopulations (Nikolic et al. 2018). Moreover, mazF overexpression differently affects cellular physiology based on the presence or absence of the MazE antitoxin that buffers MazF toxic effects (Nikolic et al. 2018). It is likewise necessary to report exact media composition used in different experiments, as well as whether bacterial populations are cultivated with or without shaking. Published experimental protocols also indicate adding the inducer of mazF expression in bacterial cultures of different optical densities, e.g., during early exponential phase (Tsilibaris et al. 2007; Nikolic et al. 2018), or mid-exponential phase (Amitai et al. 2004; Kolodkin-Gal et al. 2009). However, it is probable that the bacterial growth phase affects toxin activation due to, for instance, interaction of TA systems with mechanisms that are active during shifts in nutrient composition or during other environmental changes (Battesti et al. 2011; Wang and Wood 2011).
Measuring toxin and antitoxin levels in single cells in vivo is a challenging task. As toxin and antitoxin proteins form complexes, tagging them with fluorescent reporters would most certainly decrease stability of the complexes and prevent efficient toxin neutralization by the antitoxin. In addition, tagging toxins with fluorescent proteins could interfere with the toxin activity (Berghoff et al. 2017). Another approach is using transcriptional fluorescent reporter systems by fusing the promoter of a TA operon to a fluorescent gene reporter. However, several studies have reported that such analysis suggests weak to moderate de novo transcription of TA modules in conditions that presumably promote their activation (Shan et al. 2017; Nikolic et al. 2017; Goormaghtigh et al. 2018). One reason for the modest fluorescent readout is that many stress conditions interfere with translation, thus lowering protein synthesis, and subsequently decreasing the production of fluorescent proteins. Even though de novo transcription of TA modules is indicative of an imbalance of the toxin to antitoxin ratio and transient occurrence of free toxin proteins, transcriptional fluorescent reporters for TA systems do not directly report on toxin activation. It is therefore essential to build reporter systems that are independent of translation, and that report on toxin activation in real time.
Toxin activation influences phenotypic heterogeneity
Phenotypic heterogeneity in clonal bacterial populations can be measured between cells at a designated time point (cell-to-cell variation), or as variability in phenotypic traits of the individual cells in time (temporal variation) (Ackermann 2015). In general, negative feedback at the transcriptional level reduces cell-to-cell variation (Becskei and Serrano 2000); however, it has been suggested that negative feedback at the post-transcriptional level reduces cell-to-cell variation more efficiently than a transcriptional regulation feedback (Singh 2011). Additional interactions can change the nature of the feedback system (Ananthasubramaniam and Herzel 2014); for instance, positive feedback interactions may arise within a negative feedback motif (Bokes et al. 2018; Nikolic et al. 2018). Positive feedback amplifies phenotypic heterogeneity, and can generate subpopulations of cells with different phenotypic states. For further discussion on how different feedback loops influence cellular processes in bacterial cells, see (Smits et al. 2006; Maheshri and O’Shea 2007; Ackermann 2015).
Several previous studies indicate that the majority of chromosomally encoded type II TA systems elicit phenotypic heterogeneity in stressed bacterial populations by promoting variation in gene expression, cell size, and growth rate (Klumpp et al. 2009; Kasari et al. 2010; Nikolic et al. 2017, 2018). During ectopic MazF activation, the extent of cell-to-cell variation was slightly reduced when MazF was able to degrade its cognate transcript. Interestingly, MazF-mediated cleavage of the mazEF transcript induced pulse-like behavior and increased temporal variability in MazF levels during stress (Nikolic et al. 2018). Why would the same post-transcriptional autoregulatory circuit promote lower cell-to-cell phenotypic variation and higher temporal variability? This question requires more detailed experimental and theoretical analysis, as synthetic setups can influence the nature of feedback loops, and favor specific feedback architectures.
Current questions about mazEF (auto)regulation
The mazEF autoregulation is a complex mechanism attained by several protein–protein, protein–mRNA and protein–DNA interactions. Autoregulation of mazEF expression at the transcriptional and post-transcriptional level is responsible for fluctuations in MazF levels during ectopic stress (Nikolic et al. 2018). However, the mazG and relA transcripts are parts of the polycistronic mRNA together with mazE and mazF (Gama-Castro et al. 2016). MazG is an enzyme with pyrophosphohydrolase activity (Zhang and Inouye 2002), and it is considered to cause depletion of ppGpp (Gross et al. 2006). ppGpp is an alarmone that modulates gene expression during amino acid starvation, and it is synthesized by RelA (Dalebroux and Swanson 2012). Elucidating whether MazG plays a role in the autoregulation of the mazEFG transcriptional unit (Goormaghtigh et al. 2018), as well as how transcription of the relA-mazEF unit (Gama-Castro et al. 2016) affects mazEF expression, will provide necessary details to obtain a full picture of autoregulatory processes. Stress response mechanisms might additionally regulate mazEF expression (Battesti et al. 2011; Wang and Wood 2011). Furthermore, the MazF toxin can be inactivated by the T4 bacteriophage-dependent ADP-ribosylation (Alawneh et al. 2016; Otsuka 2016), which raises the question if MazF is chemically modified by other post-translational mechanisms. Likewise, it is still elusive whether other TA systems cross-regulate MazF activity and mazEF expression, and to what extent (Yamaguchi and Inouye 2011; Kasari et al. 2013; Wessner et al. 2015). Finally, investigating (auto)regulation of mazEF expression during physiological induction of the toxin will further determine the importance and impact on this TA system on the behavior of bacterial cells.
References
Ackermann M (2015) A functional perspective on phenotypic heterogeneity in microorganisms. Nat Rev Microbiol 13(8):497
Aizenman E, Engelberg-Kulka H, Glaser G (1996) An Escherichia coli chromosomal “addiction module” regulated by guanosine-3′5′-bispyrophosphate: a model for programmed bacterial cell death. Proc Natl Acad Sci U S A 93:6059–6063
Alawneh AM, Qi D, Yonesaki T, Otsuka Y (2016) An ADP-ribosyltransferase Alt of bacteriophage T4 negatively regulates the Escherichia coli MazF toxin of a toxin–antitoxin module. Mol Microbiol 99(1):188–198
Amitai S, Yassin Y, Engelberg-Kulka H (2004) MazF-mediated cell death in Escherichia coli: a point of no return. J Bacteriol 186:8295–8300
Ananthasubramaniam B, Herzel H (2014) Positive feedback promotes oscillations in negative feedback loops. PLoS One 9(8):e104761
Battesti A, Majdalani N, Gottesman S (2011) The RpoS-mediated general stress response in Escherichia coli.. Annu Rev Microbiol 65:189–213
Becskei A, Serrano L (2000) Engineering stability in gene networks by autoregulation. Nature 405:590–593
Berghoff BA, Wagner EG (2017) RNA-based regulation in type I toxin–antitoxin systems and its implication for bacterial persistence. Curr Genet 63(6):1011–1016
Berghoff BA, Hoekzema M, Aulbach L, Wagner EG (2017) Two regulatory RNA elements affect TisB-dependent depolarization and persister formation. Mol Microbiol 103(6):1020–1033
Bokes P, Lin YT, Singh A (2018) High cooperativity in negative feedback can amplify noisy gene expression. Bull Math Biol 80(7):1871–1899
Cataudella I, Sneppen K, Gerdes K, Mitarai N (2013) Conditional cooperativity of toxin–antitoxin regulation can mediate bistability between growth and dormancy. PLoS Comput Biol 9(8):e1003174
Christensen SK, Pedersen K, Hensen FG, Gerdes K (2003) Toxin–antitoxin loci as stress-response elements: ChpAK/MazF and ChpBK cleave translated mRNAs and are counteracted by tmRNA. J Mol Biol 332:809–819
Culviner PH, Laub MT (2018) Global analysis of the E. coli toxin MazF reveals widespread cleavage of mRNA and the inhibition of rRNA maturation and ribosome biogenesis. Mol Cell 70(5):868–880
Dalebroux ZD, Swanson MS (2012) ppGpp: magic beyond RNA polymerase. Nat Rev Microbiol 10(3):203
Fiedoruk K, Daniluk T, Swiecicka I, Sciepuk M, Leszczynska K (2015) Type II toxin–antitoxin systems are unevenly distributed among Escherichia coli phylogroups. Microbiology 161(1):158–167
Gama-Castro S, Salgado H, Santos-Zavaleta A, Ledezma-Tejeida D, Muñiz-Rascado L et al (2016) RegulonDB version 9.0: high-level integration of gene regulation, coexpression, motif clustering and beyond. Nucleic Acids Res 44(D1):D133–D143
Garcia-Pino A, Balasubramanian S, Wyns L, Gazit E, De Greve H et al (2010) Allostery and intrinsic disorder mediate transcription regulation by conditional cooperativity. Cell 142(1):101–111
Gelens L, Hill L, Vandervelde A, Danckaert J, Loris R (2013) A general model for toxin–antitoxin module dynamics can explain persister cell formation in E. coli. PLoS Comput Biol 9(8):e1003190
Gerdes K (ed) (2012) Prokaryotic toxin–antitoxins. Springer, Berlin
Gerdes K, Christensen SK, Lobner-Olesen A (2005) Prokaryotic toxin–antitoxin stress response loci. Nat Rev Microbiol 3:371–382
Goeders N, Van Melderen L (2014) Toxin–antitoxin systems as multilevel interaction systems. Toxins 6(1):304–324
Goormaghtigh F, Fraikin N, Putrinš M, Hallaert T, Hauryliuk V et al (2018) Reassessing the role of type II toxin–antitoxin systems in formation of Escherichia coli type II persister cells. mBio 9(3):e00640–e00618
Gross M, Marianovsky I, Glaser G (2006) MazG—a regulator of programmed cell death in Escherichia coli. Mol Microbiol 59(2):590–601
Harms A, Fino C, Sørensen MA, Semsey S, Gerdes K (2017) Prophages and growth dynamics confound experimental results with antibiotic-tolerant persister cells. mBio 8(6):e01964–e01917
Harms A, Brodersen DE, Mitarai N, Gerdes K (2018) Toxins, targets, and triggers: an overview of toxin–antitoxin biology. Mol Cell 70(5):768–784
Helaine S, Cheverton AM, Watson KG, Faure LM, Matthews SA et al (2014) Internalization of Salmonella by macrophages induces formation of nonreplicating persisters. Science 343(6167):204–208
Hõrak R, Tamman H (2017) Desperate times call for desperate measures: benefits and costs of toxin–antitoxin systems. Curr Genet 63(1):69–74
Kamada K, Hanaoka F, Burley SK (2003) Crystal structure of the MazE/MazF complex: molecular bases of antidote–toxin recognition. Mol Cell 11:875–884
Kasari V, Kurg K, Margus T, Tenson T, Kaldalu N (2010) The Escherichia coli mqsR and ygiT genes encode a new toxin–antitoxin pair. J Bacteriol 192:2908–2919
Kasari V, Mets T, Tenson T, Kaldalu N (2013) Transcriptional cross-activation between toxin–antitoxin systems of Escherichia coli. BMC Microbiol 13:45
Klumpp S, Zhang Z, Hwa T (2009) Growth rate-dependent global effects on gene expression in bacteria. Cell 139:1366–1375
Kolodkin-Gal I, Engelberg-Kulka H (2008) The extracellular death factor: physiological and genetic factors influencing its production and response in Escherichia coli. J Bacteriol 190(9):3169–3175
Kolodkin-Gal I, Verdiger R, Shlosberg-Fedida A, Engelberg-Kulka H (2009) A differential effect of E. coli toxin–antitoxin systems on cell death in liquid media and biofilm formation. PLoS One 4(8):e6785
Maheshri N, O’Shea EK (2007) Living with noisy genes: how cells function reliably with inherent variability in gene expression. Annu Rev Biophys Biomed 36:413–434
Marianovsky I, Aizenman E, Engelberg-Kulka H, Glaser G (2001) The regulation of the Escherichia coli mazEF promoter involves an unusual alternating palindrome. J Biol Chem 276(8):5975–5984
Masuda H, Inouye M (2017) Toxins of prokaryotic toxin–antitoxin systems with sequence-specific endoribonuclease activity. Toxins 9(4):e140
Muthuramalingam M, White JC, Bourne CR (2016) Toxin–antitoxin modules are pliable switches activated by multiple protease pathways. Toxins 8(7):e214
Nikolic N, Didara Z, Moll I (2017) MazF activation promotes translational heterogeneity of the grcA mRNA in Escherichia coli populations. PeerJ 5:e3830
Nikolic N, Bergmiller T, Vandervelde A, Albanese TG, Gelens L et al (2018) Autoregulation of mazEF expression underlies growth heterogeneity in bacterial populations. Nucleic Acids Res 46(6):2918–2931
Norton JP, Mulvey MA (2012) Toxin–antitoxin systems are important for niche-specific colonization and stress resistance of uropathogenic Escherichia coli. PLoS Pathog 8(10):e1002954
Otsuka Y (2016) Prokaryotic toxin–antitoxin systems: novel regulations of the toxins. Curr Genet 62(2):379–382
Overgaard M, Borch J, Jorgensen MG, Gerdes K (2008) Messenger RNA interferase RelE controls relBE transcription by conditional cooperativity. Mol Microbiol 69:841–857
Page R, Peti W (2016) Toxin–antitoxin systems in bacterial growth arrest and persistence. Nat Chem Biol 12:208–214
Peters JE, Thate TE, Craig NL (2003) Definition of the Escherichia coli MC4100 genome by use of a DNA array. J Bacteriol 185(6):2017–2021
Ramisetty BCM, Santhosh RS (2015) Horizontal gene transfer of chromosomal type II toxin–antitoxin systems of Escherichia coli. FEMS Microbiol Lett 363(3):fnv238
Ramisetty BCM, Santhosh RS (2017) Endoribonuclease type II toxin–antitoxin systems: functional or selfish? Microbiology 163(7):931–939
Rocker A, Meinhart A (2016) Type II toxin: antitoxin systems. More than small selfish entities? Curr Genet 62(2):287–290
Rosenfeld N, Elowitz MB, Alon U (2002) Negative autoregulation speeds the response times of transcription networks. J Mol Biol 323:785–793
Sauert M, Wolfinger MT, Vesper O, Müller C, Byrgazov K et al (2016) The MazF-regulon: a toolbox for the post-transcriptional stress response in Escherichia coli. Nucleic Acids Res 44(14):6660–6675
Schifano JM, Vvedenskaya IO, Knoblauch JG, Ouyang M, Nickels BE et al (2014) An RNA-seq method for defining endoribonuclease cleavage specificity identifies dual rRNA substrates for toxin MazF-mt3. Nat Commun 5:3538
Shan Y, Gandt AB, Rowe SE, Deisinger JP, Conlon BP et al (2017) ATP-dependent persister formation in Escherichia coli. mBio 8(1):e02267–e02216
Singh A (2011) Negative feedback through mRNA provides the best control of gene-expression noise. IEEE Trans Nanobiosci 10:194–200
Smits WK, Kuipers OP, Veening JW (2006) Phenotypic variation in bacteria: the role of feedback regulation. Nat Rev Microbiol 4:259–271
Tian C, Semsey S, Mitarai N (2017) Synchronized switching of multiple toxin–antitoxin modules by (p)ppGpp fluctuation. Nucleic Acids Res 45(14):8180–8189
Tripathi A, Dewan PC, Siddique SA, Varadarajan R (2014) MazF induced growth inhibition and persister generation in Escherichia coli. J Biol Chem 289:4191–4205
Tsilibaris V, Maenhaut-Michel G, Mine N, Van Melderen L (2007) What is the benefit to Escherichia coli of having multiple toxin–antitoxin systems in its genome? J Bacteriol 189:6101–6108
Van Melderen L (2010) Toxin–antitoxin systems: why so many, what for? Curr Opin Microbiol 13(6):781–785
Vandervelde A, Loris R, Danckaert J, Gelens L (2016) Computational methods to model persistence. In: Michiels J, Fauvart M (eds) Bacterial persistence: methods and protocols. Springer, New York, pp 207–240
Venturelli OS, Tei M, Bauer S, Chan LJ, Petzold CJ et al (2017) Programming mRNA decay to modulate synthetic circuit resource allocation. Nat Commun 8:15128
Vesper O, Amitai S, Belitsky M, Byrgazov K, Kaberdina AC et al (2011) Selective translation of leaderless mRNAs by specialized ribosomes generated by MazF in Escherichia coli. Cell 147:147–157
Wang X, Wood TK (2011) Toxin–antitoxin systems influence biofilm and persister cell formation and the general stress response. Appl Environ Microbiol 77:5577–5583
Wessner F, Lacoux C, Goeders N, d’Herouel AF, Matos R et al (2015) Regulatory crosstalk between type I and type II toxin–antitoxin systems in the human pathogen Enterococcus faecalis. RNA Biol 12(10):1099–1108
Yamaguchi Y, Inouye M (2011) Regulation of growth and death in Escherichia coli by toxin–antitoxin systems. Nat Rev Microbiol 9:779–790
Zhang J, Inouye M (2002) MazG, a nucleoside triphosphate pyrophosphohydrolase, interacts with Era, an essential GTPase in Escherichia coli. J Bacteriol 184(19):5323–5329
Zhang Y, Zhang J, Hoeflich KP, Ikura M, Quing G et al (2003) MazF cleaves cellular mRNA specifically at ACA to block protein synthesis in Escherichia coli. Mol Cell 12:913–923
Zorzini V, Buts L, Schrank E, Sterckx YG, Respondek M et al (2015) Escherichia coli antitoxin MazE as transcription factor: insights into MazE–DNA binding. Nucleic Acids Res 43(2):1241–1256
Acknowledgements
Open access funding provided by Institute of Science and Technology (IST Austria). The author is grateful to Tobias Bergmiller, Lendert Gelens, Tanino G. Albanese, Alexandra Vandervelde, Isabella Moll, and Călin C. Guet for fruitful discussions. This work has been supported by the FP7 PEOPLE, ISTFELLOW program of the IST Austria and the European Commission.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kupiec.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nikolic, N. Autoregulation of bacterial gene expression: lessons from the MazEF toxin–antitoxin system. Curr Genet 65, 133–138 (2019). https://doi.org/10.1007/s00294-018-0879-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-018-0879-8