Abstract
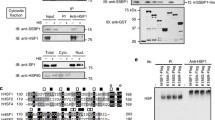
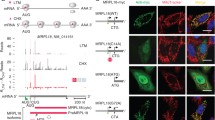
For maintenance of cytoplasmic protein quality control (PQC), cytoplasmic heat shock proteins (HSPs) negatively control heat shock transcriptional factor (HSF) in a negative feedback loop. However, how mitochondrial protein quality control (mtPQC) is maintained is largely unknown. Here we present evidence that HSF directly monitors mtPQC in the budding yeast Saccharomyces cerevisiae. Mitochondrial HSP70 (Ssc1) negatively regulated HSF activity. Importantly, HSF was localized not only in the nucleus but also on mitochondria. The mitochondrial localization of HSF was increased by heat shock and compromised by SSC1 overexpression. Furthermore, the mitochondrial protein translocation system downregulated HSF activity. Finally, mtPQC modulated the mtHSP genes SSC1 and MDJ1 via HSF, and SSC1 overexpression compromised mitochondrial function. These findings illustrate a model in which HSF directly monitors mtPQC.







Similar content being viewed by others
References
Cannino G, Di Liegro CM, Rinaldi AM (2007) Nuclear-mitochondrial interaction. Mitochondrion 7:359–366
Conn CS, Qian SB (2011) mTOR signaling in protein homeostasis: less is more? Cell Cycle 10:1940–1947
Craig EA, Gross CA (1991) Is hsp70 the cellular thermometer?. Trends Biochem Sci 16:135–140
Craig EA, Kramer J, Shilling J, Werner-Washburne M, Holmes S, Kosic-Smithers J, Nicolet CM (1989) SSC1, an essential member of the yeast HSP70 multigene family, encodes a mitochondrial protein. Mol Cell Biol 9:3000–3008
Duina AA, Kalton HM, Gaber RF (1998) Requirement for Hsp90 and a CyP-40-type cyclophilin in negative regulation of the heat shock response. J Biol Chem 273:18974–18978
Dyall SD, Brown MT, Johnson PJ (2004) Ancient invasions: from endosymbionts to organelles. Science 304:253–257
Eisenberg-Bord M, Schuldiner M (2017) Ground control to major TOM: mitochondria-nucleus communication. FEBS J 284:196–210
Endo T, Kohda D (2002) Functions of outer membrane receptors in mitochondrial protein import. Biochim Biophys Acta 1592:3–14
Fan AC, Bhangoo MK, Young JC (2006) Hsp90 functions in the targeting and outer membrane translocation steps of Tom70-mediated mitochondrial import. J Biol Chem 281:33313–33324
Gray MW (2014) The pre-endosymbiont hypothesis: a new perspective on the origin and evolution of mitochondria. Cold Spring Harb Perspect Biol 6:a016097
Haitani Y, Takagi H (2008) Rsp5 is required for the nuclear export of mRNA of HSF1 and MSN2/4 under stress conditions in Saccharomyces cerevisiae. Genes Cells 13:105–116
Haynes CM, Ron D (2010) The mitochondrial UPR—protecting organelle protein homeostasis. J Cell Sci 123:3849–3855
Haynes CM, Petrova K, Benedetti C, Yang Y, Ron D (2007) ClpP mediates activation of a mitochondrial unfolded protein response in C. elegans. Dev Cell 13:467–480
Herrmann JM, Neupert W (2000) Protein transport into mitochondria. Curr Opin Microbiol 3:210–214
Hill S, Van Remmen H (2014) Mitochondrial stress signaling in longevity: a new role for mitochondrial function in aging. Redox Biol 2:936–944
Hohfeld J, Hartl FU (1994) Role of the chaperonin cofactor Hsp10 in protein folding and sorting in yeast mitochondria. J Cell Biol 126:305–315
Hu F, Liu F (2011) Mitochondrial stress: a bridge between mitochondrial dysfunction and metabolic diseases? Cell Signal 23:1528–1533
Huh WK, Falvo JV, Gerke LC, Carroll AS, Howson RW, Weissman JS, O’Shea EK (2003) Global analysis of protein localization in budding yeast. Nature 425:686–691
Ikeda E, Yoshida S, Mitsuzawa H, Uno I, Toh-e A (1994) YGE1 is a yeast homologue of Escherichia coli grpE and is required for maintenance of mitochondrial functions. FEBS Lett 339:265–268
Kohno K (2007) How transmembrane proteins sense endoplasmic reticulum stress. Antioxid Redox Signal 9:2295–2303
Laloraya S, Gambill BD, Craig EA (1994) A role for a eukaryotic GrpE-related protein, Mge1p, in protein translocation. Proc Natl Acad Sci USA 91:6481–6485
Lasserre JP, Dautant A, Aiyar RS, Kucharczyk R, Glatigny A, Tribouillard-Tanvier D, Rytka J, Blondel M, Skoczen N, Reynier P, Pitayu L, Rotig A, Delahodde A, Steinmetz LM, Dujardin G, Procaccio V, di Rago JP (2015) Yeast as a system for modeling mitochondrial disease mechanisms and discovering therapies. Dis Model Mech 8:509–526
Mager WH, De Kruijff AJ (1995) Stress-induced transcriptional activation. Microbiol Rev 59:506–531
Mager WH, Ferreira PM (1993) Stress response of yeast. Biochem J 290:1–13
Martinus RD, Garth GP, Webster TL, Cartwright P, Naylor DJ, Hoj PB, Hoogenraad NJ (1996) Selective induction of mitochondrial chaperones in response to loss of the mitochondrial genome. Eur J Biochem 240:98–103
Mori K, Kawahara T, Yoshida H, Yanagi H, Yura T (1996) Signalling from endoplasmic reticulum to nucleus: transcription factor with a basic-leucine zipper motif is required for the unfolded protein-response pathway. Genes Cells 1:803–817
Mumberg D, Muller R, Funk M (1994) Regulatable promoters of Saccharomyces cerevisiae: comparison of transcriptional activity and their use for heterologous expression. Nucleic Acids Res 22:5767–5768
Mumberg D, Muller R, Funk M (1995) Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 156:119–122
Nakai M, Kato Y, Ikeda E, Toh-e A, Endo T (1994) Yge1p, a eukaryotic Grp-E homolog, is localized in the mitochondrial matrix and interacts with mitochondrial Hsp70. Biochem Biophys Res Commun 200:435–442
Okamoto K, Kondo-Okamoto N, Ohsumi Y (2009) Mitochondria-anchored receptor Atg32 mediates degradation of mitochondria via selective autophagy. Dev Cell 17:87–97
Pfanner N, Chacinska A (2002) The mitochondrial import machinery: preprotein-conducting channels with binding sites for presequences. Biochim Biophys Acta 1592:15–24
Qian J, Lin J, Luscombe NM, Yu H, Gerstein M (2003) Prediction of regulatory networks: genome-wide identification of transcription factor targets from gene expression data. Bioinformatics 19:1917–1926
Reading DS, Hallberg RL, Myers AM (1989) Characterization of the yeast HSP60 gene coding for a mitochondrial assembly factor. Nature 337:655–659
Richter K, Haslbeck M, Buchner J (2010) The heat shock response: life on the verge of death. Mol Cell 40:253–266
Rowley N, Prip-Buus C, Westermann B, Brown C, Schwarz E, Barrell B, Neupert W (1994) Mdj1p, a novel chaperone of the DnaJ family, is involved in mitochondrial biogenesis and protein folding. Cell 77:249–259
Saarikangas J, Barral Y (2016) Protein aggregation as a mechanism of adaptive cellular responses. Curr Genet 62:711–724
Sakurai H, Takemori Y (2007) Interaction between heat shock transcription factors (HSFs) and divergent binding sequences: binding specificities of yeast HSFs and human HSF1. J Biol Chem 282:13334–13341
Schroder M, Kaufman RJ (2005) ER stress and the unfolded protein response. Mutat Res 569:29–63
Shiota T, Imai K, Qiu J, Hewitt VL, Tan K, Shen HH, Sakiyama N, Fukasawa Y, Hayat S, Kamiya M, Elofsson A, Tomii K, Horton P, Wiedemann N, Pfanner N, Lithgow T, Endo T (2015) Molecular architecture of the active mitochondrial protein gate. Science 349:1544–1548
Sickmann A, Reinders J, Wagner Y, Joppich C, Zahedi R, Meyer HE, Schonfisch B, Perschil I, Chacinska A, Guiard B, Rehling P, Pfanner N, Meisinger C (2003) The proteome of Saccharomyces cerevisiae mitochondria. Proc Natl Acad Sci USA 100:13207–13212
Sorger PK, Pelham HR (1988) Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell 54:855–864
Tachibana T, Astumi S, Shioda R, Ueno M, Uritani M, Ushimaru T (2002) A novel non-conventional heat shock element regulates expression of MDJ1 encoding a DnaJ homolog in Saccharomyces cerevisiae. J Biol Chem 8:22140–22146
Voos W, Martin H, Krimmer T, Pfanner N (1999) Mechanisms of protein translocation into mitochondria. Biochim Biophys Acta 1422:235–254
Westermann B, Prip-Buus C, Neupert W, Schwarz E (1995) The role of the GrpE homologue, Mge1p, in mediating protein import and protein folding in mitochondria. EMBO J 14:3452–3460
Wrobel L, Topf U, Bragoszewski P, Wiese S, Sztolsztener ME, Oeljeklaus S, Varabyova A, Lirski M, Chroscicki P, Mroczek S, Januszewicz E, Dziembowski A, Koblowska M, Warscheid B, Chacinska A (2015) Mistargeted mitochondrial proteins activate a proteostatic response in the cytosol. Nature 524:485–488
Zhao Q, Wang J, Levichkin IV, Stasinopoulos S, Ryan MT, Hoogenraad NJ (2002) A mitochondrial specific stress response in mammalian cells. EMBO J 21:4411–4419
Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R (1998) Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell 94:471–480
Acknowledgements
We thank Toshiya Endo, Walter Neupert, Sabine Rospert, Bent Jakobsen, Dennis Winge, Kazutoshi Mori, Shuh-ichi Nishikawa, Takehiko Shibata, Markus Proft, Ramón Serrano, and Koji Okamoto for generous gifts of materials. We especially thank Tomohusa Tachibana and Yoshihiro Kato for execution of preliminary experiments. We give special thanks to Toshiya Endo and Shuh-ichi Nishikawa, and laboratory members of TU for helpful discussions. BY4741 was provided by the National Bio-Resource Project (NBRP) of the MEXT, Japan.
Author information
Authors and Affiliations
Contributions
TU conceived and initiated the project. TU designed the experiments. NK, YH, and TU executed the experiments and analyzed the data. NK, YH, and TU discussed the data. TU wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest were disclosed.
Additional information
Communicated by M. Kupiec.
Rights and permissions
About this article
Cite this article
Koike, N., Hatano, Y. & Ushimaru, T. Heat shock transcriptional factor mediates mitochondrial unfolded protein response. Curr Genet 64, 907–917 (2018). https://doi.org/10.1007/s00294-018-0809-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-018-0809-9