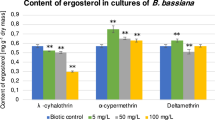
Abstract
Entomopathogenic fungi mostly attack their insect hosts by penetration through the cuticle. The outermost insect surface is covered by a lipid-rich layer, usually composed of very long chain hydrocarbons. These fungi are apt to grow on straight chain hydrocarbons (alkanes) as the sole carbon source. Insect-like hydrocarbons are first hydroxylated by a microsomal P450 monooxygenase system, and then fully catabolized by peroxisomal β-oxidation reactions in Beauveria bassiana. In this review, we will discuss lipid metabolism adaptations in alkane-grown fungi, and how an oxidative stress scenario is established under these conditions. Fungi have to pay a high cost for hydrocarbon utilization; high levels of reactive oxygen species are produced and a concomitant antioxidant response is triggered in fungal cells to cope with this drawback.


Similar content being viewed by others
References
Alconada TM, Juárez MP (2006) Acyl-CoA oxidase activity from Beauveria bassiana, an entomopathogenic fungus. J Basic Microbiol 46:435–443
Ali S, Huang Z, Li H, Bashir MH, Ren S (2013) Antioxidant enzyme influences germination, stress tolerance, and virulence of Isaria fumosorosea. J Basic Microbiol 53:489–497
Baud O, Greene AE, Li J, Wang H, Volpe JJ, Rosenberg PA (2004) Glutathione peroxidase–catalase cooperativity is required for resistance to hydrogen peroxide by mature rat oligodendrocytes. J Neurosci 4(7):1531–1540
Beers RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Bizzi A, Veneroni E, Tacconi MT, Codegoni AM, Pagani R, Cini M, Garattini S (1980) Accumulation and metabolism of uneven fatty acids present in single cell protein. Toxicol Lett 5:227–240
Boucias DG, Pendland JC (1991) The fungal cell wall and its involvement in the pathogenic process in insect hosts. In: Latge JP, Boucias DG (eds) Fungal cell wall and immune response, vol H53. Springer, Berlin, pp 303–316
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–309
Chen Q, Nijenhuis A, Preusting H, Dolfing J, Janssen DB, Witholt B (1995) Effects of octane on the fatty acid composition and transition temperature of Pseudomonas oleovorans membrane lipids during growth in two-liquid-phase continuous culture. Enzyme Microb Technol 17:647–652
Crespo R, Juárez MP, Cafferata LFR (2000) Biochemical interaction between entomopathogenous fungi and their host-like hydrocarbons. Mycologia 92:528–536
Crespo R, Juárez MP, Dal Bello GM, Padín S, Calderón Fernández G, Pedrini N (2002) Increased mortality of Acanthoscelides obtectus by alkane-grown Beauveria bassiana. Biocontrol 47:685–696
Fang GC, Hanau RM, Vaillancourt LJ (2002) The SOD2 gene, encoding a manganese-type superoxide dismutase, is upregulated during conidiogenesis in the plant-pathogenic fungus Colletotrichum graminicola. Fungal Genet Biol 36:155–165
Ferron P (1985) Fungal control. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiology, biochemistry and pharmacology. Academic Press, New York, pp 313–346
Forlani L, Juárez MP, Lavarías S, Pedrini N (2014) Toxicological and biochemical response of the entomopathogenic fungus Beauveria bassiana after exposure to deltamethrin. Pest Manag Sci 70:751–756
Freimoser FM, Hu G, St Leger RJ (2005) Variation in gene expression patterns as the insect pathogen Metarhizium anisopliae adapts to different host cuticles or nutrient deprivation in vitro. Microbiology 151:361–371
Fridovich I (1995) Superoxide radical and superoxide dismutases. Annu Rev Biochem 64:97–112
Habig W, Pabst MJ, Jakoby WB (1974) Glutathione-S-transferases. J Biol Chem 249:7130–7139
Hansberg W, Aguirre J (1990) Hyperoxidant states cause microbial cell differentiation by cell isolation from dioxygen. J Theor Biol 142(2):201–221
Inoue Y, Matsuda T, Sugiyama K, Izawa S, Kimura A (1999) Genetic analysis of glutathione peroxidase in oxidative stress response of Saccharomyces cerevisiae. J Biol Chem 274:27002–27009
Juárez MP, Crespo R, Calderón-Fernández G, Lecuona RE, Cafferata LFR (2000) Characterization and carbon metabolism in fungi pathogenic to Triatoma infestans, a Chagas disease vector. J Invertebr Pathol 76:198–207
Juárez MP, Pedrini N, Crespo R (2004) Mycoinsecticides against Chagas disease vectors: biochemistry involved in insect host hydrocarbon degradation. In: Mas-Comas S (ed) Multidisciplinarity for parasites, vectors and parasitic diseases. Monduzzi Editore, Bologna, pp 137–142
Karlsson M, Stenlid J, Olson A (2005) Identification of a superoxide dismutase gene from the conifer pathogen Heterobasidion annosum. Physiol Mol Plant Pathol 66:99–107
Ketterer B, Coles B, Meyer DJ (1983) The role of glutathione in detoxification. Environ Health Perspect 49:59–69
Lecuona RE, Clement J-L, Riba G, Joulie C, Juárez MP (1997) Spore germination and hyphal growth of Beauveria sp. on insect lipids. J Econ Entomol 89:119–123
Mantilla JG, Galeano NF, Gaitan AL, Cristancho MA, Keyhani NO, Gongora CE (2012) Comparative transcriptome analysis of the entomopathogenic fungus Beauveria bassiana grown on the coffee berry borer (Hypothenemus hampei). Microbiology 158:1826–1842
Marklund SL, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Michán S, Lledías F, Baldwin JD, Natvig DO, Hansberg W (2002) Regulation and oxidation of two large monofunctional catalases. Free Radic Biol Med 33:521–532
Michán S, Lledías F, Hansberg W (2003) Asexual development is increased in Neurospora crassa cat-3-null mutant strains. Eukaryot Cell 2(4):798–808
Michiels C, Raes M, Toussaint O, Remacle J (1994) Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free Radic Biol Med 17(3):235–248
Missall TA, Cherry-Harris JF, Lodge JK (2005) Two glutathione peroxidases in the fungal pathogen Cryptococcus neoformans are expressed in the presence of specific substrates. Microbiology 151:2573–2581
Morales Hernandez CE, Padilla Guerrero IE, Gonzalez Hernandez GA, Salazar Solis E, Torres Guzman JC (2010) Catalase overexpression reduces the germination time and increases the pathogenicity of the fungus Metarhizium anisopliae. Appl Microbiol Biotechnol 87:1033–1044
Mylonas C, Kouretas D (1999) Lipid peroxidation and tissue damage. Vivo 13(3):295–309
Napolitano R, Juárez MP (1997) Entomopathogenous fungi degrade epicuticular hydrocarbons of Triatoma infestans. Arch Biochem Biophys 334:208–214
Pedrini N, Juárez M, Crespo R, de Alaniz MJT (2006) Clues on the role of Beauveria bassiana catalases in alkane degradation events. Mycologia 98:528–534
Pedrini N, Crespo R, Juarez MP (2007) Biochemistry of insect epicuticle degradation by entomopathogenic fungi. Comp Biochem Physiol 146C:124–137
Pedrini N, Mijailovsky SJ, Girotti JR, Stariolo R, Cardozo RM, Gentile A, Juárez MP (2009) Control of pyrethroid-resistant Chagas disease vectors with entomopathogenic fungi. PLoS Negl Trop Dis 3(5):e434. doi:10.1371/journal.pntd.0000434
Pedrini N, Zhang S, Juárez MP, Keyhani NO (2010) Molecular characterization and expression analysis of a suite of cytochrome P450 enzymes implicated in insect hydrocarbon degradation in the entomopathogenic fungus Beauveria bassiana. Microbiology 156:2549–2557
Pedrini N, Ortiz-Urquiza A, Huarte-Bonnet C, Zhang S, Keyhani NO (2013) Targeting of insect epicuticular lipids by the entomopathogenic fungus Beauveria bassiana: hydrocarbon oxidation within the context of a host-pathogen interaction. Front Microbiol 4:24. doi:10.3389/fmicb.2013.00024
Saito T, Aoki J (1983) Toxicity of free fatty acids on the larval surfaces of two lepidopterous insects towards Beauveria bassiana (Bals.) and Paecilomyces fumosoroseus (Wize) Brown et Smith (Deuteromycetes: Moniliales). Appl Entomol Zool 18:225–233
Scheller U, Zimmer T, Becher D, Schauer F, Schunck W-H (1998) Oxygenation cascade in conversion of n-alkanes to α, ω-dioic acids catalyzed by cytochrome P450 52A3. J Biol Chem 273:32528–32534
Sheehan D, Meade G, Foley VM, Dowd CA (2001) Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J 360:1–16
Smith RJ, Grula A (1981) Nutritional requirements for conidial germination and hyphal growth of Beauveria bassiana. J Invertebr Pathol 37:222–230
St. Leger RJ, Cooper RM, Charnley AK (1986a) Cuticle-degrading enzymes of entomopathogenic fungi: cuticle degradation in vitro by enzymes from entomopathogens. J Invertebr Pathol 47:167–177
St. Leger RJ, Charnley AK, Cooper RM (1986b) Cuticle-degrading enzymes of entomopathogenic fungi: synthesis in culture on cuticle. J Invertebr Pathol 48:85–95
Toledo I, Noronha-Dutra AA, Hansberg W (1991) Loss of NAD(P)-reducing power and glutathione disulfide excretion at the start of induction of aerial growth in Neurospora crassa. J Bacteriol 173:3243–3249
Toledo I, Rangel P, Hansberg W (1995) Redox imbalance at the start of each morphogenetic step of Neurospora crassa conidiation. Arch Biochem Biophys 319:519–524
Wang H, Le Dall M-T, Waché Y, Laroche P, Belin J-M, Gaillardin C, Nicaud J-M (1999) Cloning, sequencing, and characterization of five genes coding for Acyl-CoA oxidase isozymes in the yeast Yarrowia lipolytica. Cell Biochem Biophys 31:165–174
Wang ZS, Gu YX, Yuan QS (2006) Effect of nutrition factors on the synthesis of superoxide dismutase, catalase, and membrane lipid peroxide levels in Cordyceps militaris mycelium. Curr Microbiol 52:74–79
Wang Z-L, Zhang L-B, Ying S-H, Feng M-G (2013) Catalases play differentiated roles in the adaptation of a fungal entomopathogen to environmental stresses. Environ Microbiol 15(2):409–418
Xiao G, Ying S-H, Zheng P, Wang Z-L, Zhang S, Xie X-Q et al (2012) Genomic perspectives on the evolution of fungal entomopathogenicity in Beauveria bassiana. Sci Rep 2:483. doi:10.1038/srep00483
Xie XQ, Ying SH, Feng MG (2010) Characterization of a new Cu/Zn-superoxide dismutase from Beauveria bassiana and two site-directed mutations crucial to its antioxidation activity without chaperon. Enzyme Microb Technol 46:217–222
Xie X-Q, Li F, Ying S-H, Feng M-G (2012) Additive contributions of two manganese-cored superoxide dismutases (MnSODs) to antioxidation, UV tolerance and virulence of Beauveria bassiana. PLoS One 7(1):e30298. doi:10.1371/journal.pone.0030298
Zhang S, Widemann E, Bernard G, Lesot A, Pinot F, Pedrini N, Keyhani NO (2012) CYP52X1, representing new cytochrome P450 subfamily, displays fatty acid hydroxylase activity and contributes to virulence and growth on insect cuticular substrates in entomopathogenic fungus Beauveria bassiana. J Biol Chem 287:13477–13486
Acknowledgments
This review article was supported in part by a grant from São Paulo Research Foundation (FAPESP) of Brazil #2014/01229-4. Original research has been supported by grants from the National Council of Scientific and Technical Research (CONICET) of Argentina (PIP0237), and the National Agency for Science and Technology Promotion of Argentina (PICT2012-1964) to N.P. C.H.B. is supported by a fellowship from the CONICET. M.P.J. and N.P. are members of the CONICET Researcher’s Career.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. E. N. Rangel.
This article is part of the Special Issue “Fungal Stress Responses”.
Rights and permissions
About this article
Cite this article
Huarte-Bonnet, C., Juárez, M.P. & Pedrini, N. Oxidative stress in entomopathogenic fungi grown on insect-like hydrocarbons. Curr Genet 61, 289–297 (2015). https://doi.org/10.1007/s00294-014-0452-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-014-0452-z